Inhibition of ethylene action by 1-methylcyclopropene
prolongs storage life of apricots
X. Fan
1, L. Argenta, J.P. Mattheis *
Tree Fruit Research Laboratory,Agricultural Research Ser6ice,US Department of Agriculture,1104N.Western A6e., Wenatchee,
WA 98801,USA
Received 10 November 1999; accepted 8 May 2000
Abstract
‘Perfection’ apricot fruit (Prunus armeniacaL.) were treated with 1ml l−11-methylcyclopropene (MCP) for 4 h at
20°C then stored at 0 or 20°C. The onset of ethylene production was delayed and respiration rate was reduced following MCP treatment. MCP treatment resulted in less firmness and titratable acidity loss during storage at both temperatures and delayed production of volatile alcohols and esters during ripening at 20°C. MCP treated fruit also exhibited less color change and were greener than controls. The effects of MCP were less pronounced following treatment of fruit at a more advanced stage of development. © Published by Elsevier Science B.V.
Keywords:Apricot; MCP; Shelf life; Respiration; Ethylene; Quality
www.elsevier.com/locate/postharvbio
1. Introduction
Apricot fruit have a very short storage life due in part to a high respiration rate and a rapid ripening process. Apricots are usually marketed soon after harvest (Hardenburg et al., 1986) but can be stored for 1 – 2 weeks at 0°C. Apricot fruit are climacteric (Biale, 1960) and the ripening pro-cess is regulated by ethylene, therefore, inhibiting ethylene biosynthesis or action should slow the ripening process. Indeed, controlled atmosphere
conditions that reduce ethylene production (Gorny and Kader, 1997) improve storage quality of apricots (Guelfat-Reich and Ben-Arie, 1967; Wankier et al., 1970; Claypool and Pangborn, 1972). Apricot fruit produce many volatile com-pounds during ripening that contribute to fruit aroma (Tang and Jennings, 1967) and the major compounds are esters (Takeoka et al., 1990). Volatile compound production is closely corre-lated to ethylene production and appears to be regulated by ethylene action in apple, plum and banana fruit (Abdi et al., 1998; Fan et al., 1998a; Golding et al., 1998). It is not clear whether production of the ester compounds is regulated by ethylene during apricot fruit ripening.
The ethylene action inhibitor, 1-methylcyclo-propene (MCP) (Sisler and Blankenship, 1996;
* Corresponding author. Tel.: +1-509-6642280; fax: + 1-509-6642280.
E-mail address:[email protected] (J.P. Mattheis). 1Present address: USDA, ARS, Eastern Regional Research Center, 600 E. Mermaid Lane, Wyndmoor, PA 19038, USA.
Sisler and Serek, 1997), has been shown to inhibit fruit ripening and improve post-storage quality of climacteric fruits (Abdi et al., 1998; Golding et al., 1998; Fan and Mattheis, 1999a; Fan et al., 1999a,b). The objective of this study was to char-acterize physiological responses of apricot fruit to MCP treatment and to evaluate fruit quality fol-lowing storage of treated fruit.
2. Materials and methods
2.1. Plant materials and quality analysis
‘Perfection’ apricots were harvested from a local commercial orchard. The fruit were segregated according to ground color: maturity 1 (M1), light green, partially turning to straw color; maturity 2 (M2), straw color on most of fruit surface. The M2 fruit were stored at 20°C for 3 additional days after harvest to allow ripening to continue and accentu-ate differences with M1 fruit. Fruit firmness was measured on two pared surfaces with a Universal TA-XT2 texture analyzer (Texture Technologies Corp. Scarsdale, NY) with a 0.79-cm tip. Measure-ment of color and titratable acidity (TA) were as reported earlier (Fan et al., 1998b). Color was measured on both the sun-exposed (red) and shade side of each fruit with a colorimeter (Minolta CR-200, Japan) fitted with CIE illuminant C and an 8-mm measuring aperture. TA was measured by titrating 5 ml juice with 0.1 N KOH to pH 8.2 and was expressed as mM H+. MCP (BioTechnology
for Horticulture, Burr Ridge, IL) treatments were applied at 1ml l−1MCP for 4 h at 20°C in a sealed
230-l steel chamber. Control fruit were treated similarly only without MCP. After treatment, M1 and M2 fruit were stored in perforated plastic box liners at 20°C for 14 days or 1 (M1 only), 3 or 5 weeks at 0°C plus 3 days at 20°C. There were 30 fruit per maturity per treatment for each quality attribute measured, except ten replicates of three fruit were used for analysis of TA.
2.2. Effects of MCP on apricot respiration and
ethylene production
Control and MCP treated fruit (five to six fruit,
total weight approximately 0.5 kg, four replicates per treatment) were placed into 12-l chambers purged with air at 6 l h−1. Respiration rate was determined by measuring the CO2concentration in the outflow from each chamber. A 1-ml gas sample was withdrawn from the outflow, and CO2 in the sample was determined using a HP 5890 gas chromatograph (Hewlett Packard, Avondale, PA) equipped with a methanizer (John T. Booker, Austin, TX) and a 60-cm stainless steel column (2-mm ID) packed with Porapak Q (80/100 mesh). Gas flows for N2, H2, and air were 65, 30, and 300 ml min−1
, respectively. Oven, injector, and FID temperatures were 30, 50, and 200°C, respectively. Ethylene was measured by injecting 0.5-ml gas samples from the chamber outflows into a gas chromatograph (HP 5880A, Hewlett Packard, Avondale, PA) fitted with a 30-cm glass column (3.2 mm ID) packed with Porapak Q (80/100 mesh). Gas flows for N2, H2, and air were 30, 30, and 300 ml min−1, respectively. Oven, injector, and FID temperatures were 50, 50, and 200°C. CO2 and C2H4 were measured every 1 or 2 days.
2.3. Effect of MCP on production of apricot
6olatile compounds
(J&W Scientific, 60 m×0.25 mm i.d., 0.25 mm
film thickness). Conditions for chromatography were: initial oven temperature 35°C held for 5 min, increased to 50°C at 2°C min−1
, increased to 200°C at 5°C min−1
and held 5 min. Linear velocity of the He carrier gas was 30 cm s−1. Mass spectra were obtained by electron ionization at 70 eV. Transfer line and ion source tempera-tures were 280 and 180°C, respectively. Com-pound identification was made by comparison of spectra of sample compounds with those con-tained in the Wiley – NBS library and by compar-ing retention indices of sample compounds and
standards. Quantification was performed using selected ion monitoring for base peaks, and quan-titative values were calculated using response fac-tors generated with standards.
2.4. Statistical analysis
Analysis of variance, GLM, and LSD proce-dures were performed using SAS (SAS Institute, Cary, NC). Orthogonal comparisons were per-formed on the data from the storage study. First, coefficients of polynomials were generated using the Proc IML procedure, and then significance of polynomials was calculated using the generated coefficients in Contrast statements of the GLM procedure.
3. Results
3.1. Effects of MCP on apricot respiration and
ethylene production
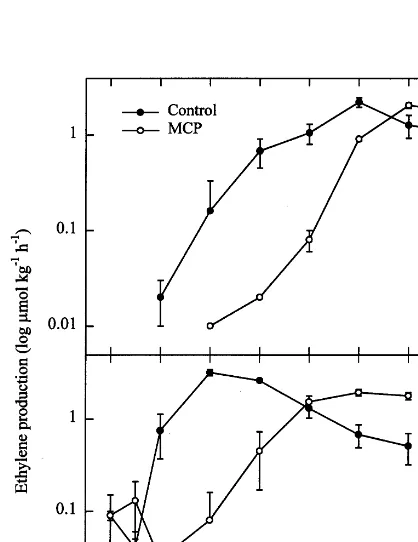
Ethylene production by M1 control apricots increased beginning 2 days after treatment and reached a maximum on day 10 (Fig. 1A). The onset of the ethylene climacteric and maximum ethylene production for MCP treated fruit were delayed 4 and 2 days, respectively, compared to controls. Ethylene production by M2 control fruit reached a maximum 4 days after treatment while MCP treated fruit had a delayed ethylene climac-teric and lower maximum production (Fig. 1B). Respiration rate of both M1 and M2 controls increased initially and reached maximum produc-tion after 10 (M1) or 8 (M2) days (Fig. 2A and B). MCP treatment resulted in reduced respiration rate throughout the post-treatment investigation period although respiration rate of treated fruits approached those of controls after 14 and 12 days for M1 and M2 fruit respectively.
3.2. Effect of MCP on apricot 6olatile compound
production
Volatile compounds identified in headspace from intact apricot fruit were esters, alcohols, aldehydes, ketones and acetic acid. The major
Fig. 2. Respiration rate of ‘Perfection’ apricot fruit during ripening at 20°C. Fruit were segregated at harvest into two groups, maturity 1 (M1), light green, partially turning to straw color; or maturity 2 (M2), straw color on most of fruit surface. Fruit from each maturity group were treated with 1ml l−1 MCP for 4 h at 20°C, then held at 20°C for 14 (A:M1) or 12 (B:M2) days. Error bars represent S.D. of means of four replicate samples consisting of 10 – 11 whole fruit (approxi-mately 1 kg fresh weight). When bars are absent, the value for S.D. was within the dimensions of the symbol.
MCP treatment (data not shown). Production of total esters and alcohols (primarily ethanol, butanol and hexanol) increased during ripening, however, fruit treated with MCP had reduced ester and alcohol production throughout the investigation period (Fig. 3A and B).
3.3. Effects of MCP on apricot shelf life
After 10 days at 20°C, M1 fruit treated with MCP had significantly higher firmness and TA compared to controls (Table 1). No significant weight loss occurred during storage at 20 or 0°C (data not shown). The MCP treated fruit also had higher hue (greener) and lower chroma surface
Fig. 3. Production of volatile esters and alcohols by ‘Perfec-tion’ apricot fruit during ripening at 20°C. Samples were collected from maturity one fruit that had a light green, partially turning to straw color at harvest. The fruit were treated with 1 ml l−1 MCP for 4 h at 20°C. Error bars represent S.D. of means of four replicate samples consisting of 10 – 11 whole fruit (approximately 1 kg fresh weight). When bars are absent, the value for S.D. was within the dimensions of the symbol.
volatile compounds were (by decreasing amount produced): butyl butanoate, hexyl butanoate, de-canal, hexyl 2-methylbutanoate, hexyl hexanoate, hexyl acetate, pentyl butanoate, butyl acetate, butyl hexanoate, nonanal, butyl propanoate, octanal and butyl 2-methylbutanoate. Most of the
headspace volatile compounds were esters.
Emission of the ketones 6-methyl 5-hepten 2-one, acetone as well as acetic acid did not change during ripening, and the amounts of these compounds were not affected by MCP treatment
(data not shown). Production of aldehydes
Table 1
Effect of MCP on apricot qualitya
Initial 10 days after treatment
M2c M1
M1b M2
Ck MCP Ck MCP
16.3b 2.1b
Firmness N 41ad 4.9a 3.1b 7.5a
268b 218b
TA mM H+ 303a 251a 224b 254a
85.4b 69.3b 73.1a
96.1a 73.3a
Hue 74.6a
43.4a 43.6a
Chroma 43.0a 42.1b 44.1a 43.8a
aFruit were segregated at harvest based on ground color: maturity 1 (M1), light green, partially turning to straw color; maturity 2 (M2), straw color on most of fruit surface. Fruit treated with MCP were exposed to 1ml l−1MCP for 4 h. Controls (Ck) and treated fruit (MCP) were then stored at 20°C for 10 days prior to analysis. Analysis of firmness and color (hue and chroma) were conducted using 30 single fruit replicates. Soluble solids content (SSC) and titratable acidity (TA) were measured using ten replicate juice samples each prepared using three fruit.
bFruit analyzed the day of harvest.
cFruit analyzed after harvest plus 3 days at 20°C.
dMeans with same letter are not significantly different (Fisher’s LSD,P50.05).
color values. The M2 fruit treated with MCP also
had higher firmness compared to controls,
however, no difference in TA was detected. No treatment effects on surface color were observed. The difference in firmness between control and MCP treated M2 fruit was not as pronounced as observed with M1 fruit.
3.4. Effects of MCP on apricot storage quality
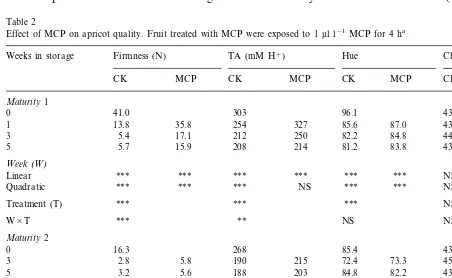
Firmness of both control and MCP treated fruit decreased as storage duration increased (Table 2). MCP treated fruit had significantly higher firmness compared to controls after all storage durations for both M1 and M2 fruit. Differences between controls and MCP treated fruit were smaller for M2 compared to M1 fruit. MCP treated fruit had higher TA compared to controls regardless of storage duration or initial maturity. Fruit hue values decreased as storage duration increased however, MCP treated M1 fruit had higher hue values compared to controls, indicating that the fruit were greener than the controls. No treatment difference in hue for M2 fruit was observed. No consistent change in chroma was detected in relation to storage dura-tion or treatment. Significant linear and quadratic effects for storage duration indicated firmness,
TA, hue and chroma decreased with increased storage duration. The linear component demon-strates the linear regression of quality parameters. The quadratic component measures the additional improvement due to fitting the second-order poly-nomial and indicates that the amount of decrease in these quality attributes increases with storage duration.
4. Discussion
Production of esters and alcohols increased during the ripening of control M1 fruit over a 9-day period at 20°C. The increase in ester and alcohol production during ripening is also typical for apples, another climacteric fruit that produces esters as ripening proceeds (Dirink et al., 1989; Mattheis et al., 1991; Song and Bangerth, 1996). The increase in production of ethylene, esters, and alcohols by M1 apricot fruit during ripening at 20°C is delayed following treatment with MCP. Production of volatile compounds during climac-teric fruit ripening is inhibited following exposure to MCP (Song et al., 1997; Abdi et al., 1998; Golding et al., 1998; Fan and Mattheis, 1999b), indicating a role for ethylene in regulation of volatile production during climacteric fruit ripen-ing (Fan et al., 1998a; Wyllie et al., 1998). Al-though production of esters and alcohols by MCP treated apricot fruit increased following an
in-crease in ethylene production 4 days after treat-ment, the amount of esters and alcohols produced was less than the amount produced by control fruit over the 9-day period when volatile tion was characterized. The lack of full produc-tion of these groups of volatile compounds by apricot fruit after ethylene production increased by MCP treated fruit is similar to results previ-ously reported for banana fruit (Golding et al., 1998). Their results indicated that while initiation of ripening-related volatile synthesis was depen-dent on climacteric ethylene production, no direct relationship between production rates of ethylene and volatile compounds was evident. A close as-sociation with respiration rate and volatile pro-duction has been established for apple fruit (Song and Bangerth, 1996) and reduced respiration rate is a possible factor limiting volatile production rate by fruit treated with MCP (Golding et al.,
Table 2
Effect of MCP on apricot quality. Fruit treated with MCP were exposed to 1ml l−1MCP for 4 ha
Weeks in storage Firmness (N) TA (mM H+) Hue Chroma
CK MCP CK MCP CK MCP CK MCP
Maturity1
0 41.0 303 96.1 43.0
35.8 254 327 85.6 87.0 43.4
1 13.8 43.4
1998). The increase in respiration rate by M1 apricots treated with MCP was delayed 8 days, therefore at 9 days after MCP treatment when apricot volatile production was measured, respira-tion rate was still lower compared to control fruit. The largest proportion of volatile compounds detected in this study were esters and alcohols; however, an appreciable amount of aldehydes were also produced. The lack of quantitative changes in aldehyde production during ripening of apricots is similar to the pattern observed during apple fruit ripening (Mattheis et al., 1991). Aldehydes are readily converted to alcohols and esters in ripening fruit (Yamashita et al., 1977; DePooter et al., 1987). When the capacity to produce esters is fully developed by the presence of alcohol dehydrogenase and alcohol acyl co-A reductase (Fellman and Mattheis, 1995), alde-hydes may be converted to alcohols at a rate that prevents aldehyde accumulation in excess of the concentration present in immature fruit. Esters that contribute to the odor of intact apricots include: ethyl butanoate, ethyl 2-methylbutanoate, butyl butanoate, ethyl hexanoate, ethyl 2-methylbutanoate, and hexyl 2-methylbutanoate (Takeoka et al., 1990). Because treatment of apri-cot fruit with MCP at 1ml l−1 delays production
of esters, development of characteristic apricot aroma following MCP treatment may also be delayed. Although MCP treated M1 fruit were significantly firmer compared to controls after 10 days at 20°C following treatment, firmness of treated fruit was low (4.9 N). Considering the treated fruit were quite soft 10 days after treat-ment and that ester and alcohol production was significantly less than controls 9 days after treat-ment, it is possible that the delay in ester produc-tion may preclude development of typical aroma while the fruit have acceptable firmness.
Although fruit quality is enhanced by harvest at an advanced developmental stage (Kader et al., 1977), logistics of harvest, packing and transport typically require producers to harvest less than fully mature fruit. The M2 fruit used in this study were overmature for commercial storage due to relatively low firmness values. Riper fruit were used to study whether MCP can effectively slow ripening of tree ripe fruit that have already
devel-oped good eating quality. The results indicate MCP treatment slowed firmness and TA loss in fruit from both maturities although the retention of firmness and TA was less with M2 fruit. Fruit treated with MCP had higher TA compared to controls and could increase the sourness of treated fruit. The potential for increased sourness along with delayed production of compounds that contribute to aroma warrant thorough sensory evaluation of MCP-treated fruit prior to commer-cial use of this material for apricots.
In summary, MCP delays the climacteric rise in respiration and ethylene production during apri-cot fruit ripening. MCP treatment also slowed the changes in firmness, TA and color associated with fruit ripening. MCP can effectively prolong the storage of apricots at both 20 and 0°C, but assess-ment of consumer acceptance of MCP treated fruit should be determined prior to commercial use.
Acknowledgements
The authors thank Dave Buchanan and Janie Gausman for excellent technical assistance and BioTechnology for Horticulture for providing EthylBloc.
References
Abdi, N., McGlasson, W.B., Holford, P., Williams, M., Mizrahi, Y., 1998. Response of climacteric and suppressed-climacteric plums to treatment with propylene and 1-methylcylcopropene. Postharvest Biol. Technol. 14, 29 – 39. Andrich, G., Fiorentini, R., 1986. Effects of controlled atmo-sphere on the storage of new apricot cultivars. J. Sci. Food Agric. 37, 1203 – 1208.
Biale, J.B., 1960. Respiration of fruits. In: Ruhland, W. (Ed.), Encyclopedia of Plant Physiology, vol. 12. Springer, Berlin, pp. 536 – 592.
Byers, R.E., 1997. Peach and nectarine fruit softening follow-ing aminoethoxyvinylglycine sprays and dips. HortScience 32, 86 – 88.
Claypool, L.L., Pangborn, R.M., 1972. Influence of controlled atmosphere storage on quality of canned apricots. J. Am. Soc. Hort. Sci. 97, 636 – 638.
Dirink, P., De Pooter, H., Schamp, N., 1989. Aroma develop-ment in ripening fruits. Am. Chem. Soc. Symp. Ser. 388, 23 – 34.
Fan, X., Mattheis, J.P., 1999a. Methyl jasmonate promotes degreening of apple fruit independent of ethylene action. HortScience 34, 310 – 312.
Fan, X., Mattheis, J.P., 1999b. Impact of 1-methylcyclo-propene and methyl jasmonate on apple volatile produc-tion. J. Agric. Food Chem. 47, 2847 – 2853.
Fan, X., Mattheis, J.P., Buchanan, D., 1998a. Continuous requirement of ethylene for apple fruit volatile synthesis. J. Agric. Food Chem. 46, 1959 – 1963.
Fan, X., Mattheis, J.P., Fellman, J.K., 1998b. Responses of apples to postharvest jasmonate treatments. J. Am. Soc. Hort. Sci. 123, 421 – 425.
Fan, X., Blankenship, S., Mattheis, J.P., 1999a. MCP inhibits apple fruit ripening. J. Am. Soc Hort. Sci. 124, 690 – 695. Fan, X., Mattheis, J.P., Blankenship, S., 1999b. Development
of superficial scald, coreflush, and peel greasiness is re-duced by MCP. J. Agric. Food Chem. 47, 3063 – 3068. Fellman, J.K., Mattheis, J.P., 1995. Ester biosynthesis in
rela-tion to harvest maturity and controlled-atmosphere storage of apples. Am. Chem. Soc. Symp. Ser. 596, 149 – 163. Golding, J.B., Shearer, D., Wyllie, S.G., McGlasson, W.B.,
1998. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol. Technol. 14, 87 – 98.
Gorny, J.R., Kader, A.A., 1997. Low oxygen and elevated carbon dioxide atmospheres inhibit ethylene biosynthesis in pre-climacteric and climacteric apple fruit. J. Am. Soc. Hort. Sci. 122, 542 – 546.
Guelfat-Reich, S., Ben-Arie, R., 1967. Different factors affect-ing the keepaffect-ing quality of ‘Canino’ apricots in cold storage. In: Proceedings of the 12th International Congress on Refrigeration, vol. 3, pp. 447 – 457.
Hardenburg, R.E., Watada, A.E., Wang, C.Y., 1986. The commercial storage of fruits, vegetables, and florist and nursery stocks. U.S. Department of Agriculture Agricul-ture Handbook. No. 66.
Kader, A.A., Stevens, M.A., Albright-Holton, M., Morris, L.L., Algazi, M., 1977. Effect of fruit ripeness when picked
on flavor and composition in fresh market tomatoes. J. Am. Soc. Hort. Sci. 102, 724 – 731.
Kader, A.A., El-Goorani, M.A., Sommer, N.F., 1982. Postharvest decay, respiration, ethylene production, and quality of peaches held in controlled atmospheres with added carbon monoxide. J. Am. Soc. Hort. Sci. 107, 856 – 859.
Mattheis, J.P., Fellman, J.K., Chen, P.M., Patterson, M.E., 1991. Changes in headspace volatiles during physiological development of Bisbee Delicious apple fruit. J. Agric. Food Chem. 39, 1902 – 1906.
Sisler, E.C., Blankenship, S.M., 1996. Method of counteract-ing an ethylene response in plants. U.S. Patent No. 5 518 988
Sisler, E.C., Serek, M., 1997. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Phys-iol. Plant. 100, 577 – 582.
Song, J., Bangerth, F., 1996. The effect of harvest date on aroma compound production from ’Golden Delicious’ ap-ple fruit and relationship to respiration and ethylene pro-duction. Postharvest Biol. Technol. 8, 259 – 269.
Song, J., Tian, M.S., Dilley, D.R., Beaudry, R.M., 1997. Effect of 1-MCP on apple fruit ripening and volatile production. HortScience 32, 536.
Takeoka, G.R., Flath, R.A., Mon, T.R., Teranishi, R., Guentert, M., 1990. Volatile constituents of apricot (Prunus armeniaca). J. Agric. Food Chem. 38, 471 – 477. Tang, C.S., Jennings, W.G., 1967. Volatile compounds of
apricot. J. Agric. Food Chem. 15, 24 – 28.
Wankier, B.N., Salunkhe, D.K., Campbell, W.F., 1970. Effects of controlled atmosphere storage on biochemical changes in apricot and peach fruit. J. Am. Soc. Hort. Sci. 95, 604 – 609.
Wyllie, G.S., Golding, J.B., McGlasson, W.B., Williams, M., 1998. The relatinship between ethylene and aroma volatiles porduciton n climacteric ripening fruit. Dev. Food Sci. 40, 375 – 384.
Yamashita, I., Iino, K., Nemoto, Y., Yoshikawa, S., 1977. Studies on flavor development in strawberries. 4. Biosythe-sis of volatile alcohol and esters from aldehyde during ripening. J. Agric. Food Chem. 25, 1165 – 1168.