Summary Single cells were mechanically isolated from leaf-derived callus of mature Juniperus oxycedrus L. These cells divided and gave rise to callus when plated on medium con-taining growth regulators. Best plating efficiency was obtained on a modified Schenk and Hildebrandt medium supplemented with 0.6 µM 2,4-dichlorophenoxyacetic acid and 100 mg l−1 casein hydrolyzate. Although single-cell-derived callus showed poor morphogenic potential, both adventitious shoots and embryogenic tissues differentiated from the callus. We also achieved induction of somatic embryogenesis in leaf explants of mature J. oxycedrus trees cultured in the presence of 6.0 or 10.0 µM 2,4-dichlorophenoxyacetic acid or picloram. Fre-quency of embryogenic callus ranged from 6 to 18%; however, under the culture conditions tested, isolated embryos failed to develop into plants.
Keywords: adventitious budding, mature explants, somatic em-bryogenesis.
Introduction
Many conifer species have been micropropagated by axillary shoot proliferation, adventitious shoot organogenesis or so-matic embryogenesis (for review see Harry and Thorpe 1994). However, the application of tissue culture technology to tree improvement still suffers from three serious limitations (Aboel-Nil 1987): (1) low reproductive efficiency and high cost; (2) difficulty in applying the techniques to mature trees; and (3) difficulty in regenerating plants from callus and cell suspension cultures, a prerequisite for research on genetic engineering and biotechnology.
The induction of shoot organogenesis or somatic embryo-genesis from isolated needles would permit the cloning of aged and selected trees. There have been few reports, however, of regeneration from somatic tissues of mature conifers (Park and Bonga 1993, Attree and Fowke, 1993). Gómez and Segura (1994) successfully regenerated adventitious shoots from leaves of mature Juniperus oxycedrus L. (Cupressaceae), sug-gesting that the maturation process did not significantly affect the morphogenetic potential of isolated tissues from this spe-cies. Thus, Juniperus species may provide the opportunity to test whether cells from explants are competent to undergo morphogenesis through other developmental pathways.
The aim of this research was to develop a method for the isolation and culture of single cells from leaf-derived callus of adult J. oxycedrus. The embryogenic capacity of leaf explants was also studied. Juniperus oxycedrus was chosen for this study because the species has good potential for reforestation of degraded Mediterranean areas.
Material and methods
Plant material
Terminal shoots from lateral branches (5 cm length) were collected from several 30-year-old Juniperus oxycedrus trees growing in their natural habitat in La Eliana, Valencia, Spain. The shoots were surface sterilized by a 15-min immersion in 1% NaOCl containing 0.01% Tween-20, followed by four 5-min washes with sterile distilled water. Subsequently, shoot tips (2 mm in length) were excised from long terminal shoots and cultured on basal medium SH (see below). Leaves (0.5 cm in length) of the last pair of verticils, isolated from shoots cultured for 2 months on the SH medium, were used as the source of explants for the experiments.
Culture media and incubation conditions
The two basal media used in the experiments were modified MS medium with MS macronutrients (Murashige and Skoog 1962) and modified SH medium with SH macronutrients (Schenk and Hildebrandt 1972). Both media included MS micronutrients, vitamins as described by Vieitez et al. (1985), 3% sucrose, and 0.7% Difco-Bacto agar, at pH 5.8. Growth regulators were added to the media before autoclaving for 20 min at 120 °C.
Unless otherwise stated, cultures were incubated in growth chambers at 26 ± 2 °C in a 16-h photoperiod supplied by Sylvania (GTE Gro-lux, F36W/GRO, Germany) fluorescent tubes (80 µmol m−2 s−1 photon flux at culture level). Single-cell cultures were maintained in continuous darkness at 27 ± 1 °C.
Growth regulators and inhibitors were obtained from Sigma Chemical Company, USA. All other chemicals were of analyti-cal grade.
Morphogenesis in leaf and single-cell cultures of mature
Juniperus
oxycedrus
M. P. GOMEZ and J. SEGURA
Departamento de Biología Vegetal, Facultad de Farmacia, Universidad de Valencia, Av. Vicent Andrés Estellés s/n, 46100-Burjasot, Valencia, Spain
Received July 20, 1995
Callus induction
Excised leaves (adaxial surface to the medium) were cultured in 15 × 100 mm petri dishes sealed with Parafilm, containing 30 ml of modified MS or SH medium supplemented with 0.06, 0.6 or 6.0 µM 2,4-dichlorophenoxyacetic acid (2,4-D), in com-bination with 0, 0.05, 0.5, 5.0 or 10.0 µM benzyl adenine (BA) or kinetin. Each treatment contained five replications (five dishes each with five explants). Morphogenic responses were evaluated after 45 days. Subsequently, calli were transferred to fresh growing medium for another 45 days. This procedure was repeated until the cultures were 135 days old.
Isolated cell culture
Leaf-derived calli that had been cultured for 135 days on modified SH medium with 6 µM 2,4-D, were used as the source of cells. For cell isolation, samples of calli (about 2 g fresh weight) were transferred to 125-ml flasks containing 30 ml of liquid basal SH or MS medium and agitated at 110 rpm for 24 h on a horizontal rotatory shaker. The resulting cell suspensions were filtered through a stainless steel sieve (0.062 mm) and centrifuged for 5 min at 100 g. Isolated cells were resuspended in medium and the cell density determined with a Fuchs-Rosenthal haemocytometer. Cells were cultured in 35-mm plastic dishes (Corning, New York) sealed with Parafilm, following the plating technique of Bergmann (1960). Each dish contained 1 ml of solidified SH or MS medium (0.5 ml of cell suspension + 0.5 ml of 1.4% agar medium). In all experiments, a culture density of 1 × 104 cells ml−1 was employed.
In the first experiment, modified SH or MS medium was supplemented with 0.0, 0.5 or 5.0 µM BA or kinetin; and 0.0 or 0.6 µM 2,4-D or naphthalene acetic acid (NAA). In sub-sequent experiments, cell cultures were established on modi-fied SH medium with 0.6 µM 2,4-D. In these experiments the effects of: (a) a nurse callus; (b) conditioned medium; and (c) 100 mg l−1 casein hydrolyzate (CH) or 5.0 mM glutamine were studied. Casein hydrolyzate was added to the medium before autoclaving. Glutamine was sterilized by filtration through a Millipore filter. All treatments were replicated five times.
For nurse callus cultures, samples (2 mm in diameter) of leaf-derived calli, induced in the presence of 6 µM 2,4-D, were deposited on the surface of the plated cells. To prepare condi-tioned medium, freshly isolated J. oxycedrus callus cells (5 × 104 cells ml−1) were incubated for 4 days in modified SH liquid medium (100 rpm) supplemented with 0.6 µM 2,4-D. Sub-sequently, cells were discarded by centrifugation (5 min at 100 g) and the supernatant filtered through a Millipore filter and mixed with the isolated cells before plating.
After 30 days of culture, plating efficiency ((No. of colonies and microcalli per dish)/(No. of cellular units per dish) × 100) was determined by estimating microcalli and colonies com-posed of not less than 30 cells.
Morphogenesis in callus derived from single-cell cultures
Calli derived from single cells were plated on modified SH medium supplemented with 0.6 µM 2,4-D and 100 mg l−1 CH. After 30 days of culture, thin layers of solidified medium
bearing colonies and microcalli were transferred to 60-mm petri dishes containing 5 ml of the same medium and main-tained for 30 days in darkness or in a 16-h photoperiod. Subsequently, calli with a minimum diameter of 2 mm were individually cultured in glass tubes (150 × 25 mm) containing 20 ml of modified SH medium with 100 mg l−1 CH, 0.5, 1.0 or 5.0 µM BA, kinetin or zeatin, and 0.06 or 0.6 µM IBA, NAA or 2,4-D. Morphogenic responses were recorded after 90 days. Each treatment contained 12 replications. Calli showing cau-logenic or embryogenic responses were transferred to modi-fied SH medium without growth regulators.
Finally, single-cell-derived calli were cultured for 90 days in 90-mm petri dishes containing 30 ml of modified SH medium supplemented with 100 mg l−1 CH, 0.6 µM NAA, 0.5 µM kinetin and 10−6, 10−5 or 10−4 M colchicine or trigonelline or 1, 10 or 50 M actinomicyn D. Colchicine, trigonelline and actinomycin D were sterilized by filtration through a Millipore filter. Each treatment contained three replications (three dishes, each with nine calli).
Somatic embryogenesis from cultured leaves
Leaf explants were cultured in 90-mm petri dishes containing 30 ml of modified SH medium with 6.0 or 10.0 µM 2,4-D or picloram. The dishes, sealed with Parafilm, were maintained in darkness or in a 16-h photoperiod. Each treatment contained 10 replications (ten dishes, each with five explants). After 45 days, explants were transferred to fresh medium for another 45 days. After this time, the percentage of calli showing embryos was evaluated. Calli showing embryos (or the isolated somatic embryos) were subcultured on modified SH medium with or without 0.6 µM 2,4-D or 0.5 µM BA.
Statistical analysis
Significance of treatment effects was determined by analysis of variance (Statgraphics program, Version 6, Manugistics, Rockville, MD), employing a completely random design. Per-centage data were subjected to arcsine transformation before analysis. Variation among treatment means were analyzed by Tukey’s procedure (1953). All experiments were conducted at least twice.
Results and discussion
Morphogenesis in callus cultures
regulator were selected for cell isolation because of their size, friable texture and absence of necrosis.
Media containing cytokinins promoted adventitious bud dif-ferentiation from cultured leaf explants. Maximal bud differ-entiation rates occurred on modified SH medium supplemented with BA. The presence of 2,4-D reduced the cytokinin-induced caulogenesis. After the first 45 days of culture, most of the induced shoots did not grow more than 5 mm, and explants died within 90 days of transfer to fresh medium.
After the first transfer to fresh medium, a small percentage (3--5%) of the explants grown on modified SH medium with 6.0 µM 2,4-D developed a white and translucent embryogenic-like callus.
Isolated cell culture
The procedure used for cell isolation from leaf-derived calli gave cell populations of 95 to 100% single cells as determined microscopically, and no clumps greater than four cells were observed. Yield of cells isolated from 1 g of leaf-derived callus was 4.3 × 104.
In the treatments where growth occurred, cultured cells began to divide within 4--7 days, giving rise to colonies of eight to 10 cells after 10 days of culture. Many of these
colonies developed into microcalli that were microscopically visible after 20--30 days.
Cell survival and division strongly depended on the presence of auxin in the culture medium. No cell proliferation was observed in cultures without growth regulators or supple-mented only with cytokinin (Table 1). The auxin 2,4-D was more effective than NAA in promoting cell division, especially when cultures were established on modified SH medium. Cy-tokinins significantly reduced auxin-induced cell proliferation. This antagonistic effect was particularly evident when the cells were plated on modified SH medium. The highest plating efficiency was achieved on modified SH medium supple-mented with 0.6 µM 2,4-D (Table 1). In protoplasts of Picea glauca (Moench) Voss, 2,4-D also promoted cell division and this effect was inhibited by BA or kinetin (Bekkaoui et al. 1987).
To determine optimal culture conditions for callus growth, isolated cells were plated on modified SH medium with 0.6 µM 2,4-D, a nurse callus, and either CH, glutamine or conditioned medium. As shown in Table 2, the effects of the growth adjuvants depended mainly on the presence of a nurse callus. Thus, in cultures without nurse callus, the plating effi-ciency was significantly increased by the addition of CH. In contrast, glutamine or the conditioned medium decreased this
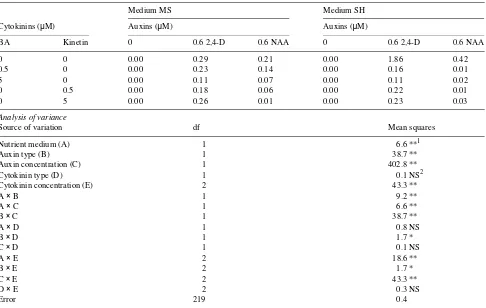
Table 1. Plating efficiencies (%, (No. of colonies and microcalli per dish)/(No. of cellular units per dish)100) of isolated cells from leaf-derived calli of mature Juniperus oxycedrus, cultured at 1 × 104 cells ml−1 for 30 days in modified MS or SH medium supplemented with auxins and cytokinins. Data are mean values of five replications.
Medium MS Medium SH
Cytokinins (µM) Auxins (µM) Auxins (µM)
BA Kinetin 0 0.6 2,4-D 0.6 NAA 0 0.6 2,4-D 0.6 NAA
0 0 0.00 0.29 0.21 0.00 1.86 0.42
0.5 0 0.00 0.23 0.14 0.00 0.16 0.01
5 0 0.00 0.11 0.07 0.00 0.11 0.02
0 0.5 0.00 0.18 0.06 0.00 0.22 0.01
0 5 0.00 0.26 0.01 0.00 0.23 0.03
Analysis of variance
Source of variation df Mean squares
Nutrient medium (A) 1 6.6 **1
Auxin type (B) 1 38.7 **
Auxin concentration (C) 1 402.8 **
Cytokinin type (D) 1 0.1 NS2
Cytokinin concentration (E) 2 43.3 **
A × B 1 9.2 **
A × C 1 6.6 **
B × C 1 38.7 **
A × D 1 0.8 NS
B × D 1 1.7 *
C × D 1 0.1 NS
A × E 2 18.6 **
B × E 2 1.7 *
C × E 2 43.3 **
D × E 2 0.3 NS
Error 219 0.4
parameter in the presence of a nurse callus. Nevertheless, these nurse cultures significantly increased the plating efficiency of the control and promoted the formation of macroscopically visible calli (1--3 mm in diameter) when the medium was supplemented with CH or glutamine (Table 2).
Favorable effects of a nurse callus on cell division and callus formation have been reported in protoplast cultures of Pinus sylvestris L. (Hohtola and Kvist 1991). The addition of CH or glutamine to the culture medium generally favors growth, especially when the assimilative mechanisms of nitrate and ammonium are not operative (Kirby et al. 1987). In some cultures, however, amino acids either repress nitrate reductase or decrease the free endogenous hormonal concentrations by conjugation, thus inhibiting cell division and growth (Marion-Poll and Caboche 1984). The presence of a nurse callus in single cell cultures of J. oxycedrus could potentiate some of these effects, decreasing plating efficiencies in cultures supple-mented with conditioned medium or glutamine. Favorable effects of glutamine and CH on cell division and growth have been reported on callus (Durzan and Chalupa 1976), cell (Dur-zan and Chalupa 1976, Kirby 1982) and protoplast (Kirby 1980, Bekkaoui et al. 1987, Lainé et al. 1988) cultures of different conifers. In contrast, CH was either ineffective or it inhibited growth in cell suspension cultures of Pseudotsuga menziesii (Mirb.) Franco (Kirby et al. 1987). Conditioned medium has been found to overcome the inhibited growth of some cells at low densities (George 1993).
Morphogenesis in callus derived from single cells
Single-cell-derived colonies and microscopically visible mi-crocalli were transferred to fresh medium and incubated for 30 days in darkness or in a 16-h photoperiod. This procedure
favored further callus formation and yielded an average of 20--30 calli per plate with a diameter of between 2 and 4 mm. Photoperiod did not significantly affect the number and growth of the calli (data not shown); therefore, all regeneration experi-ments were accomplished with calli grown in a 16-h photo-period.
Calli grew in size when transferred to several regeneration media. Maximal growth responses were obtained in the pres-ence of 0.6 µM 2,4-D (data not shown). Growth regulator requirements for cell division in J. oxycedrus cultures differed between isolated cells and their derived callus. Cytokinins alone were ineffective for cell cultures (Table 1), whereas cytokinins promoted cell division in cell-derived calli, al-though the higher concentrations of zeatin and kinetin caused necrosis of some calli.
After 60 days of culture, three of the calli grown in the presence of 0.6 µM NAA and 5.0 µM of BA developed a green color and meristemoids and buds appeared on their surface. Nevertheless, only one shoot, 2--3 cm long, regenerated per organogenic explant. Within the same culture time, some of the calli cultured on media with NAA and kinetin or zeatin com-binations (0.06 µM NAA and 1.0 µM kinetin; 0.6 µM NAA and 0.5 µM kinetin; 0.6 µM NAA and 1.0 µM kinetin; 0.6 µM NAA and 1.0 µM zeatin) differentiated embryogenic callus and somatic embryos. The overall percentage of embryogenic cultures ranged from 8 to 16%. The isolated embryos failed to develop into plants when transferred to basal medium without growth regulators.
The addition of colchicine, trigonelline or actinomycin D to the culture medium did not affect callus growth (data not shown), and none of these compounds promoted the differen-tiation of organized structures. In cultures of Nicotiana, Datura and Brassica these three inhibitors favored the forma-tion of adventitious buds (Sethi et al. 1990).
Under the culture conditions tested, single-cell-derived calli of J. oxycedrus showed poor morphogenic capacity. At pre-sent, callus-mediated plant regeneration plays no role in the clonal propagation of many conifers (for review see Jelaska 1987, Mehra-Palta and Thompson 1987, Aboel-Nil 1987). Also, with few exceptions, it has been difficult to maintain conifer callus through several subcultures (Harry and Thorpe 1994). In addition to the rapid loss of morphogenic capacity, the chances of genetic instability are increased (Patel and Berlyn 1982). However, Gladfelter and Phillips (1987) re-ported that a longer duration of frequent subcultures increased organogenic responses from callus of Pinus eldarica.
Somatic embryogenesis in leaf cultures
Because 3--5% of the calli cultured on modified SH medium with 6.0 µM 2,4-D developed a white and translucent embryo-genic-like callus, we examined the embryogenic potential of cultured leaf explants of J. oxycedrus.
After 45 days of culture, callus proliferated from the cut ends and abaxial surfaces of the leaf explants. All concentra-tions of 2,4-D and picloram tested promoted callus formation and their effects were not substantially modified by photope-riod. Most of the calli were friable and yellowish in color.
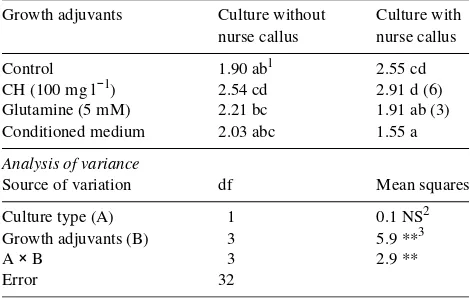
Table 2. Effects of casein hydrolyzate (CH), glutamine or conditioned medium and nurse callus on plating efficiency (%) of isolated cells from leaf-derived calli of mature Juniperus oxycedrus, cultured at 1 × 104 cells ml−1 for 30 days in modified SH medium containing 0.6 µM 2,4-D (Control). Values in parenthesis are the number of macroscopi-cally visible calli. Data are mean values of five replications.
Growth adjuvants Culture without Culture with nurse callus nurse callus
Control 1.90 ab1 2.55 cd CH (100 mg l−1) 2.54 cd 2.91 d (6) Glutamine (5 mM) 2.21 bc 1.91 ab (3) Conditioned medium 2.03 abc 1.55 a
Analysis of variance
Source of variation df Mean squares
Culture type (A) 1 0.1 NS2 Growth adjuvants (B) 3 5.9 **3
A × B 3 2.9 **
Error 32
1 Values followed by the same letter are not significantly different
according to Tukey’s test at P = 0.05.
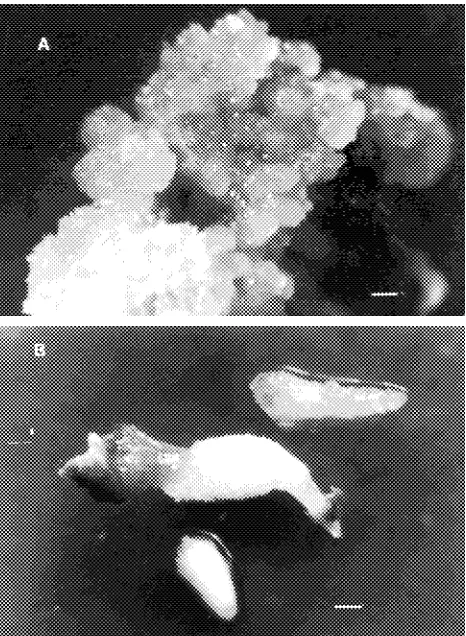
Nevertheless, about 18% of the calli induced in the presence of 6.0 µM picloram in light were friable with white and translu-cent zones, which coincides with the described characteristics for embryogenic callus in conifers (Attree et al. 1991). Explant subculture caused necrosis and death of some calli, especially those grown in the presence of picloram or 10.0 µM 2,4-D. However, all tested treatments induced embryogenic callus formation after 90 days of culture (Table 3, Figure 1A).
Embryogenic calli contained numerous embryos at different stages of development. Some had discernible roots and hypo-cotyls, but most somatic embryos exhibited marked atrophy of the cotyledons (Figure 1B). Isolated embryos failed to develop into plants when transferred to modified SH medium without growth regulators or supplemented with 0.6 µM 2,4-D or 0.5 µM BA. Within 30 days of culture on these media, both embryogenic calli and embryos died. The cause of such rapid senescence was not determined, although we obtained prelimi-nary evidence of the presence of non-functional meristems on these embryos; however, other factors such as suboptimal culture conditions and use of explants from mature trees could not be discounted. Factors controlling conversion of conifer somatic embryos have recently been reviewed (Attree and Fowke 1993). The embryogenic potential of isolated organs is affected by the ontogenic age of the tissues (Ruaud et al. 1992). With few exceptions (Ruaud et al. 1992, Chavez et al. 1992, Westcott 1994), plant regeneration through somatic embryo-genesis in conifers has only been obtained with immature or mature zygotic embryos (Tautorus et al. 1991, Attree and Fowke 1993); the use of other explants, even those derived from 10--20-day-old seedlings, was found to limit the embryo-genic process (Attree and Fowke 1991, Berlyn et al. 1991).
Our results show that leaf-derived callus is a good material for isolation and culture of cells from adult J. oxycedrus, but more research is needed to improve the morphogenic compe-tence of the cell-derived callus. We also successfully induced somatic embryogenesis in leaf explants from mature trees of J. oxycedrus; however, the frequency of leaf explants forming embryos was low and we were unable to identify conditions that promoted embryo conversion into plants.
Acknowledgments
This work was supported by the Conselleria de Cultura, Educación y Ciencia de la Generalitat Valenciana, grant to P.G.
References
Aboel-Nil, M.N. 1987. Tissue culture of Douglas-fir and Western North American Conifers. In Cell and Tissue Culture in Forestry, Vol. 3. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff Publish-ers, Dordrecht, The Netherlands, pp 80--100.
Attree, S.M. and L.C. Fowke. 1991. Micropropagation through so-matic embryogenesis in conifers. In Biotechnology in Agriculture and Forestry, Vol. 17, High-tech and Micropropagation I. Ed. Y.P.S. Bajaj. Springer-Verlag, Berlin, pp 53--70.
Attree, S.M. and L.C. Fowke. 1993. Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult. 35:1--35.
Bekkaoui, F., P. Saxena, S.M. Atree, L.C. Fowke and D.I. Dunstan. 1987. The isolation and culture of protoplasts from an embryogenic cell suspension culture of Picea glauca (Moench) Voss. Plant Cell Rep. 6:476--479.
Bergmann, L. 1960. Growth and division of single cells of higher plants in vitro. J. Gen. Physiol. 43:841--851.
Berlyn, G.P., S.J. Kohls and A.O. Anoruo. 1991. Caribbean pine (Pinus caribea Morelet). In Biotechnology in Agriculture and For-estry, Vol. 16, Trees III. Ed. Y.P.S. Bajaj. Springer-Verlag, Berlin, 254--268.
Chavez, V.M., R.E. Litz, P.A. Moon and K. Norstog. 1992. Somatic embryogenesis from leaf callus of mature plants of the gymno-sperm Ceratozamia mexicana var. Robusta (Miq.) Dyer (Cy-cadales). In Vitro Cell. Dev. Biol. 28P:59--63.
Table 3. Embryogenic callus from leaf explants of mature Juniperus oxycedrus cultured on modified SH medium with 2,4-D or picloram. Cultures were maintained for 90 days in darkness or in a 16-h photo-period. Data are mean values of 10 replications.
Growth regulators (µM) % Embryogenic callus
Photoperiod Darkness
2,4-D 6.0 8 (96)1 6 (80)1 10.0 6 (40) 8 (40) Picloram 6.0 18 (30) 10 (50) 10.0 6 (20) 6 (50)
1 Values in parentheses are the percentage of explants with callus.
Durzan, D.J. and V. Chalupa. 1976. Growth and metabolism of cells and tissues of jack pine (Pinus banksiana). I. The establishment and some characteristic of a proliferated callus from jack pine seedlings. Can. J. Bot. 54:468--482.
George, E.F. 1993. Plant propagation by tissue culture, Part 1. Exeget-ics Limited, Edington, Wilts, England, 574 p.
Gladfelter, H.J. and G.C. Phillips. 1987. De novo shoot organogenesis of Pinus eldarica Medw in vitro. I. Reproducible regeneration from long-term callus cultures. Plant Cell Rep. 6:163--166.
Gómez M.P. and J. Segura. 1994. Factors controlling adventitious bud induction and plant regeneration in mature Juniperus oxycedrus
leaves cultured in vitro. In Vitro Cell. Dev. Biol. 30P:210--218. Harry, I.S. and T.A. Thorpe. 1994. In vitro culture of forest trees. In
Plant Cell and Tissue Culture. Eds. I.K. Vasil and T.A. Thorpe. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 539--560.
Hohtola, A. and A.P. Kvist. 1991. Preparation of protoplasts from callus derived from buds of mature Scots pine and subsequent induction of cell proliferation. Tree Physiol. 8:423--428.
Jelaska, S. 1987. European pines. In Cell and Tissue Culture in Forestry, Vol. 3. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp 42--60.
Kirby E.G. 1980. Factors affecting proliferation of protoplasts and cell cultures of Douglas-fir. In Plant Cell Cultures: Results and Perspec-tives. Eds. F. Sala, B. Parisi, R. Cella and O. Cifieri. Elsevier/North Holland, Amsterdam, pp 289--293.
Kirby E.G. 1982. The effect of organic nitrogen sources on growth of cell cultures of Douglas-fir. Physiol. Plant. 56:114--119.
Kirby, E.G., T. Leustek and M.S. Lee. 1987. Nitrogen nutrition. In Cell and Tissue Culture in Forestry, Vol. 1. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp 67--88.
Lainé, E., H. David and A. David. 1988. Callus formation from cotyledon protoplasts of Pinus oocarpa and Pinus patula. Physiol. Plant. 72:374--378.
Marion-Poll, A. and A. Caboche. 1984. Relationships between auxin and amino acid metabolism of tobacco protoplast-derived cells. Plant Physiol. 75:1048--1053.
Mehra-Palta, A. and D.G. Thompson. 1987. Tissue culture of Eastern North American conifers. In Cell and Tissue Culture in Forestry, Vol. 3. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff Publish-ers, Dordrecht, The Netherlands, pp 61--79.
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473--497.
Park, Y.S. and J.M. Bonga. 1993. Conifer micropropagation: its func-tion in tree improvement programs. In Micropropagation of Woody Plants. Ed. M.R. Ahuja. Kluwer Academic, Dordrecht, The Nether-lands, pp 457--470.
Patel, K.R. and G.P. Berlyn. 1982. Genetic instability of multiple buds of Pinus coulteri regenerated from tissue culture. Can. J. For. Res. 12:93--101.
Ruaud, J.N., J. Bercetche and M. Pâques. 1992. First evidence of somatic embryogenesis from needles of 1-year-old Picea abies
plants. Plant Cell Rep. 11:563--566.
Schenk, R.V. and A.C. Hildebrandt. 1972. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50:199--204.
Sethi, U., A. Basu and S.G. Mukherjee. 1990. Role of inhibitors in the induction of differentiation in callus cultures of Brassica, Datura
and Nicotiana. Plant Cell Rep. 8:598--600.
Tautorus, T.E., L.C. Fowke and D.I. Dunstan. 1991. Somatic embryo-genesis in conifers. Can. J. Bot. 69:1873--1899.
Tukey, J.V. 1953. Some selected quick and easy methods of statistical analysis. Trans. NY Acad. Sci. Ser. II. 16:88--97.
Vieitez, A.M., M.C. San José and E. Vieitez. 1985. In vitro plantlet regeneration from juvenile and mature Quercus robur L. J. Hortic. Sci. 60:99--106.