Summary To assess the influence of stream water on leaf gas exchange and water potential in different sized boxelder trees (Acer negundo L.), we compared these characteristics in trees growing beside a perennial stream and a nearby ephemeral stream in a montane--riparian forest in northern Arizona. Pat-terns of tree water use were quantified by stable isotope analy-sis (δ18O). Physiological characteristics were similar for large
and small trees. Similarity between sites in predawn and day-time water potentials and xylem δ18O indicated that stream water was not a physiologically important water source. Sea-sonal and site variations in light-saturated net photosynthetic rate were significantly related to leaf-to-air vapor pressure deficit (r = --0.691) and foliar nitrogen concentration (r = 0.388). Although deep water was the dominant water source, surface soil water was utilized following precipitation, espe-cially by small trees. We conclude that net carbon gain and severity of water stress are only weakly coupled to stream water availability.
Keywords: Acer negundo, nitrogen, photosynthesis, riparian forests, stable isotopes, stomatal conductance, water relations.
Introduction
Riparian forests are a threatened ecosystem in the American Southwest (Lowe and Brown 1982, Johnson and Haigt 1984). Decline of native riparian trees may result from modification of surface and ground water flows by impoundments and diversions, as well as by changes in associated abiotic and biotic factors, such as increased alluvium salinity and invasion by nonnative species (e.g., Stromberg and Patten 1990, Smith et al. 1991, Busch et al. 1992, Busch and Smith 1993, Busch and Smith 1995).
Linkages between water sources, water use, and productiv-ity of riparian trees in the American Southwest are poorly understood. Many riparian trees of western North America are assumed to be phreatophytes that avoid water stress by tapping reliable water sources with deep roots. However, riparian trees can be subject to water stress, especially in mid to late summer when water availability is reduced ( Dina and Klikoff 1973, Foster and Smith 1991, Smith et al. 1991, Dawson and Ehler-inger 1993a). Stream flow diversions have been associated
with detrimental physiological changes and reduced growth of riparian trees in western North America (Stromberg and Patten 1990, Smith et al. 1991, Tyree et al. 1994). The effects of stream diversion on leaf physiology can differ among trees of different sizes, with greater detrimental effects reported for juvenile trees than for mature trees (Smith et al. 1991). Juve-nile trees may be especially sensitive to water stress because shallow root systems limit their water source to the surface soil, whereas more reliable water sources in deep soil and ground water are only available to deeply rooted, larger trees (Dawson and Ehleringer 1991, Dawson 1993).
In this study, we compared water sources, leaf gas exchange and water relations of different-sized boxelder (Acer neg-undo L.) trees growing beside a perennial stream and a nearby ephemeral stream in a montane--riparian forest in northern Arizona. Specifically, we assessed: (1) the effect of stream water accessibility on water-source utilization, water relations and photosynthesis of different-sized trees; (2) if tree size and dependability of the water source influence shifts in water-source use during the growing season; and (3) the roles of environmental and physiological factors in limiting photosyn-thesis of boxelder under field conditions.
Materials and methods
Study sites
The study area was in upper Oak Creek Canyon in north-cen-tral Arizona (35°00′ N, 111°44′ W). Oak Creek is a perennial stream that drains the southern edge of the Colorado Plateau south of Flagstaff, Arizona. Annual precipitation in the region averages 57 cm (Schubert 1974), occurring as snow and rain during winter (November--March) and as monsoonal thunder-showers during late summer (July--September). Upper Oak Creek Canyon contains a cold-temperate, montane--riparian forest type (Brown 1982). Dominant tree species include: white fir (Abies concolor (Gord.) Lindl. ex Hildebr.), boxelder (Acer negundo), bigtooth maple (Acer grandidentatum Marsh.), Arizona alder (Alnus oblongifolia Torr.), velvet ash (Fraxinus velutina Torr.), Arizona walnut (Juglans major (Torr.) A. Heller), Gambel oak (Quercus gambelii Nutt.), pon-derosa pine (Pinus ponderosa Dougl. ex P. Laws & C. Laws),
Boxelder water sources and physiology at perennial and ephemeral
stream sites in Arizona
THOMAS E. KOLB,
1STEPHEN C. HART
1and RONALD AMUNDSON
21
School of Forestry, College of Ecosystem Science and Management, Northern Arizona University, Flagstaff, AZ 86011-5018, USA
2 Division of Ecosystem Sciences, University of California, Berkeley, CA 94720, USA
Received March 15, 1996
Arizona sycamore (Platanus wrightii P. Wats.), black cherry (Prunus serotina J.F. Ehrh.) and narrowleaf cottonwood (Populus angustifolia James).
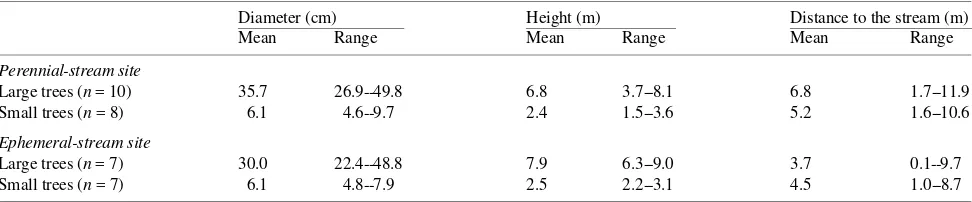
We measured water-source use and physiological charac-teristics of boxelder trees at two upper Oak Creek Canyon sites differing in proximity to perennial stream water. The perennial stream site is located adjacent to Oak Creek, and the ephemeral stream site is located adjacent to Ritter Wash, an intermittent stream that drains into Oak Creek. The ephemeral stream site is 0.4 km from the perennial stream site, and 0.2 km upstream from the intersection of Ritter Wash and Oak Creek. Both sites have similar elevation (1,650 m), slope and aspect. Although stream water can occur at the ephemeral stream site in early spring following snowmelt, surface water at the ephemeral stream site was not observed during the study (April--Novem-ber 1994). This observation, coupled with the distance be-tween the ephemeral stream site and perennial water in Oak Creek, strongly suggest that there was no direct uptake of stream water by vegetation at the ephemeral stream site.
Soils from both sites are previously unclassified types in the Soil Taxonomy Family of sandy-skeletal, mixed, mesic Typic Udifluvents (Soil Survey Staff 1990, S.C. Hart, personal obser-vation 1994). Despite depths of several meters, these soils have high rates of water infiltration and low capacities for water storage because of their coarse texture, and because ≥ 35% of their volume is occupied by rock fragments (particles greater than 2 mm in diameter).
Site environment
At both sites, we measured volumetric soil water content weekly between late April and early October 1994, with a time-domain reflectometry system (TRASE System 1, Soil-moisture Corp., Santa Barbara, CA). We established sample areas at each site every 10 m along a linear transect in the riparian forest at a distance of 2.3 m from the stream bank edge. A pair of stainless steel rods was inserted into the mineral soil to a depth of 30 cm at each of three sample areas at both sites. A regression relationship between volumetric soil water con-tent and the dielectric constant of soil between the pair of rods was applied to predict soil water content at each site. The regression relationship was determined for soils from each site in a laboratory experiment in which soils wetted to field capac-ity were air dried (sensu, Gray and Spies 1995). The same linear regression equation provided a good fit to data for soils from both sites (r = 0.982).
At one sample location for each site, we measured soil (15 and 30 cm depths) and air temperatures (1 m above ground) hourly over the same timespan described for soil water content measurements. Temperatures were measured with thermocou-ples and recorded with dataloggers (Campbell Scientific Inc., Logan, UT). A tipping-bucket gauge was used to measure daily bulk precipitation from a clearing near the perennial stream site.
Isotopic analysis of tree water sources
At both sites, we sampled ground water, stream water, soil water, and xylem water monthly during the 1994 growing
season. Ground water was sampled from springs adjacent to the perennial stream site (Cave Spring) and in the upper drain-age of the ephemeral stream site (Ritter Spring). Stream water was sampled from well-mixed sections of the stream. Ground and stream water samples were collected in glass vials fitted with air-tight polyethylene caps and stored at 4 °C. Because the ephemeral stream did not flow during the study period, no stream water samples were obtained from this site.
Soil was sampled along the same 30-m transects described above. Samples were pooled over the 0--30 cm soil layer at the first sampling date (May 2). Thereafter, the 0--5 cm and 5--30 cm soil layers were sampled separately. Soil samples were placed in air-tight jars and stored at --18 °C until water was extracted. Several 5- to 7-cm long suberized stem sections (Dawson and Ehleringer 1993b) were sampled from the same three large and three small boxelder trees at each site on each sampling date. All of these trees were growing within 3 m of the stream bank, and were chosen from the population of trees sampled for physiological measurements (Table 1). Suberized stems were placed in air-tight jars and stored at --18 °C. Water from soil and xylem samples was extracted by a cryogenic vacuum distillation technique (Sternberg et al. 1986, Ehleringer and Osmond 1989, Smith et al. 1991).
Precipitation samples from significant individual storm events (> 5 mm) were collected in 10 polyethylene bottles in an open area adjacent to the perennial stream site. Precipitation collections represent over 95% of the precipitation volume that occurred during the study period. A small amount of mineral oil was contained in each bottle to prevent evaporation of collected water between sample bottle placement and retrieval (0.5 to 3 days, depending on storm duration). Precipitation water was stored at --18 °C.
All water samples were analyzed for 18O/16O isotope ratio at Lawrence Berkeley National Laboratory, Berkeley, CA, on a V6 Prism Isotope Ratio Mass Spectrometer by an automated CO2--H2O equilibration technique (Epstein and Mayeda 1953).
Oxygen isotope ratios (δ18O values) were expressed as:
δ18
O =(Rsample⁄Rstandard−1)1000‰,
where R refers to the 18O/16O ratios of sample and standard (standard mean ocean water, SMOW), respectively. Duplicate isotopic analyses of randomly selected water samples (run about every 25 samples) always differed by ≤ 0.1‰.
We applied a two-member mixing model to calculate the relative proportion of surface soil (0--30 cm) to deep soil or ground water utilized by each tree size at each site (White 1989). Because ground and stream water were isotopically similar (see Results), we could not distinguish between these two water sources at the perennial-stream site. We assumed that when tree xylem and ground and stream water δ18O were
was uniformly utilized by tree roots. This assumption was not rigorously tested; however, we did observe living fine roots at all depths between 1 and 30 cm.
Tree water potential and gas exchange
We measured xylem water potential and leaf gas exchange on both large and small boxelder trees (Table 1) at each site. All sample trees grew in a closed-canopy forest within 12 m of the stream bank. The light environment for large and small trees at both sites was heterogeneous as a result of shading by other trees. Most small trees at both sites likely originated as sprouts from older stems that had been damaged by flooding. Although we are aware of potential differences in leaf physiology be-tween male and female boxelder trees (Dawson and Ehleringer 1993a), sex determination of sample trees was not possible because neither flowers nor seeds were present.
Xylem water potential and light-saturated leaf gas exchange characteristics were measured approximately every two weeks from time of full-leaf expansion in spring (mid May) to onset of leaf senescence in fall (early September). Net photosyn-thetic rate and stomatal conductance to water vapor were measured midmorning (0900--1100 h) on all dates, and early afternoon (1300--1500 h) on the first five dates. After mid July, afternoon measurements of gas exchange were not made be-cause of frequent precipitation and cloudy conditions. At each date and sampling time within a date, a different sample of three to five trees from each site and tree size class was measured to minimize defoliation. At each sampling time within a date, the order of measurement between sites and tree sizes within sites was determined randomly.
We measured gas exchange on one representative leaf per tree in the lower or middle canopy exposed to photosyntheti-cally active radiant flux that was more than saturating for boxelder (> 600 µmol m−2 s−1, Foster 1992). Gas exchange was measured over 30 s on a fixed leaf area (8.2 cm2) with an
LI-6200 portable photosynthesis system (Li-Cor, Inc., Lin-coln, NE) equipped with a 250-ml cuvette. During measure-ments, water vapor pressure and temperature in the cuvette were within 10% and 3 °C of ambient values, respectively. For large trees, we made approximately 90% of measurements in situ on attached leaves, and about 10% of measurements were made on leaves from twigs detached with pole pruners because of difficulty in accessing the leaves for in situ measurements. Leaves on detached twigs were measured within 30 s
follow-ing detachment in a light environment similar to that in the canopy. Preliminary studies showed that net photosynthetic rates of leaves on detached twigs do not differ from those of leaves attached to the tree under our experimental conditions. Similar methods have been used successfully in studies with other species (e.g., Gower et al. 1993, Meng and Arp 1993).
Leaf-to-air vapor pressure deficit was calculated as the dif-ference between atmospheric vapor pressure measured in the cuvette and saturated vapor pressure calculated for leaf meso-phyll. Leaf temperature used in calculating saturated vapor pressure of leaf mesophyll was measured with a fine-wire thermocouple placed on the leaf underside during the gas exchange measurement. Short-term measurements of leaf tem-perature with an external thermocouple may have underesti-mated leaf temperature, and consequently vapor pressure deficit, compared with estimates made by permanently at-tached in situ thermocouples (Tyree and Wilmot 1990).
Mid-morning and early afternoon xylem water potentials were measured with a pressure chamber (Model 1000 PMS Instruments, Corvallis, OR), as described by Ritchie and Hinckley (1975), on the trees sampled for gas exchange assess-ments. Predawn xylem water potential (0500--0600 h) was measured on all dates in five trees from each site and size class. Preliminary studies showed that a more precise endpoint as-sessment was obtained with terminal twig segments than with leaf rachises because the large twig cross-sectional area facili-tated visual observation, and because there was less phloem flow from twigs compared with leaf rachises. Therefore, mid-morning and afternoon assessments of xylem water potential were made on the terminal portion of the twig supporting the leaf sampled for the gas exchange measurement. Predawn assessments were made on a similar twig portion.
Foliar nitrogen
We measured total nitrogen concentration of leaves from five trees sampled for gas exchange measurements, for each com-bination of date, site and tree size class. Leaf tissue was ground (< 0.85 mm) and digested by a micro-Kjeldahl method (Issac and Johnson 1976), which oxidizes organic nitrogen to ammo-nium. Ammonium concentration was measured with a Lachat flow-injection analyzer (QuikChem Systems 1992). Nitrogen concentration was calculated on a leaf area basis (g cm−2) to facilitate comparison with leaf area-based gas exchange data.
Table 1. Observed mean and range of stem diameter (1 cm above soil level), stem height, and distance from stream bank of large and small boxelder trees.
Diameter (cm) Height (m) Distance to the stream (m)
Mean Range Mean Range Mean Range
Statistical analysis
We compared differences in soil water content between sites at each date by a one-way ANOVA. Mid-morning and afternoon net photosynthetic rates, predawn, mid-morning, and after-noon water potentials, leaf nitrogen concentration, and δ18O of stem water were compared between sites and tree sizes for each date with a fixed effects, two-factor ANOVA. Factors in the ANOVA were site, tree size class, and their interaction. A fixed effects, two-factor ANOVA was also conducted for each sampling date to test for significant differences in δ18O values of soil water extracted from each site at 0--5 cm and 5--30 cm soil depths (except on May 2 when soil was collected from the 0--30-cm depth), with soil depth, site, and their interaction as factors. Ground and stream waters sampled on a given date were generally within the analytical precision of oxygen iso-topic measurements (about 0.2‰), so δ18O values of ground water and stream water were pooled by date and tested for significant variation over the growing season by a one-factor ANOVA. Broad-scale relationships between net photosyn-thetic rate and environmental and physiological variables were
examined with scatter plots and Pearson-product correlations from data pooled over sites and tree sizes. Statistical analyses were performed with the SAS software package (SAS Insti-tute, Cary, NC) and Statgraphics Statistical Software (STSC, Rockville, MD).
Results
Site environment
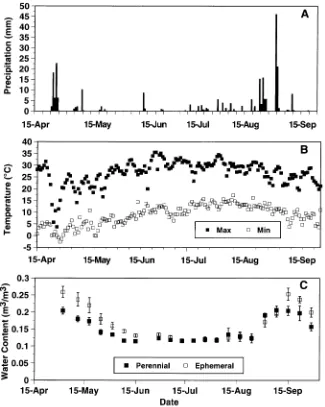
At both sites, soil water content decreased from late April to mid June, remained relatively constant until mid August and increased for several weeks in September (Figure 1). Soil water content was significantly higher at the ephemeral stream site than at the perennial-stream site during the wet conditions prevailing in April and early May, but there were no significant differences between sites under drier conditions. High soil water content was associated with periods of frequent, heavy precipitation (Figure 1). Daily maximum air temperature gen-erally increased between mid April and mid June, and
creased between mid August and late September (Figure 1). During the study, freezing temperatures occurred occasionally until mid May, but not thereafter (Figure 1).
Tree water sources
The δ18O of ground and stream water averaged --12.0‰
be-tween April and October, with no significant monthly variation (Figure 2). The δ18O of precipitation increased from --13.6‰ in late April to about --3.3‰ in mid June. Precipitation δ18O remained fairly constant between mid June and late August, and then decreased to --7.6‰ at the end of the growing season in September (Figure 2).
At both sites, δ18O of soil water at both soil depths exhibited a temporal trend similar to that of precipitation (Figure 2). In early May, δ18O of soil water in the 5--30 cm depth was similar
to that of ground and stream water. Subsequently, δ18O of soil water in this layer gradually increased, reaching a maximum value of about --3.5‰ at both sites in early September. Soil
water δ18O generally decreased at the end of the growing
season following several large precipitation events (Figure 1), because of relatively low δ18O precipitation values (Figure 2). The δ18O of soil water from the 0--5 cm soil depth was
significantly greater than from the 5--30 cm depth on all sampling dates except September 9. Soil water δ18O at both soil depths differed significantly between the perennial- and ephemeral-stream sites only on May 15 and August 10. Soil water δ18O was higher at the perennial-stream site than at the ephemeral-stream site on May 15, whereas the pattern was the opposite on August 10. There were no significant soil depth by site interactions on any sampling date.
On all sampling dates, δ18O of xylem water did not differ significantly between large and small trees or between sites, and there were no significant tree size by site interactions. Xylem water δ18O was similar to ground and stream water early in the growing season to early August (Figure 2). From early August until the end of the growing season, xylem water δ18O increased steadily to reach values between those of
ground/stream water and precipitation.
Based on the mixing model (Table 2), large trees at the perennial-stream site were exclusively dependent on stream or ground water, or both, until fall (September and October) when up to 20% of water uptake was from surface soil. Small trees at the perennial-stream site used surface soil water in both early summer and fall. Both large and small trees at the ephem-eral-stream site used surface soil water early and late in the growing season. At both sites, small trees depended on surface soil water more frequently and in greater proportion than large trees (Table 2). When surface soil water was used, it generally represented < 40% of total water uptake.
Tree water potential
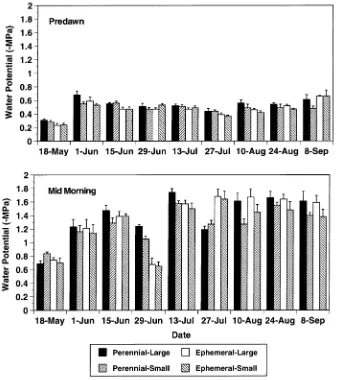
On average, predawn water potential varied from about --0.3 to --0.5 MPa, with the highest values in May (Figure 3). Predawn
Table 2. Estimated percentage of xylem water of large and small boxelder trees derived from surface soil (0--30 cm) at perennial- and ephemeral-stream sites. Values were calculated by a two end-member mixing model, assuming that surface soil water (0--30-cm depth) and deep soil or ground water were the only water sources. Where xylem and ground water δ18O values were not statistically different (two-tailed Student’s t-test, P > 0.05), the percentage of xylem water derived from soil was assumed to be zero.
Sampling date Percentage of xylem water derived from surface soil
Perennial-stream site Ephemeral-stream site
Large Small Large Small
May 2 0 0 28.2 43.6
May 18 0 0 17.3 55.4
June 15 0 10.4 0 0
July 14 0 8.4 0 0
August 10 0 0 0 0
Sept 9 15.6 16.8 24.0 31.8
Oct 6 20.0 38.6 34.1 40.0
water potentials did not differ significantly between large and small trees on any date. Likewise, there was no significant interaction between site and tree size on predawn water poten-tial. Predawn water potential was similar at each site on five dates (Figure 3). On the other four measurement dates (May 18, June 15, July 27, August 10), predawn water poten-tials were significantly lower at the perennial-stream site than at the ephemeral-stream site; however, these differences were small (approximately 0.1 MPa) (Figure 3).
On average, mid-morning water potentials varied from about --0.7 MPa in May to about --1.5 MPa from July through September (Figure 3). There was no significant interaction between site and tree size on mid-morning water potential on any date. Mid-morning water potentials were significantly lower at the perennial- versus the ephemeral-stream site only on June 29 and July 13, and the opposite pattern was observed on July 27. There was no significant effect of tree size on mid-morning water potential, except on August 10 when large trees had lower water potentials than small trees. On all dates, afternoon water potentials were within 0.2 MPa of mid-morn-ing water potentials and there were no significant site or size effects (data not shown).
Net photosynthetic rate
A significant interaction between site and tree size on net photosynthetic rate was only observed at mid-morning on July 13. Mid-morning photosynthetic rates did not differ sig-nificantly between sites or tree sizes between May 18 and June 15 (Figure 4); however, between June 29 and September 8, mid-morning photosynthetic rates were generally greater at the perennial stream site than the ephemeral-stream site, with significant differences observed on July 27 and September 8. Large trees had significantly greater mid-morning photosyn-thetic rates than small trees only on August 10 and Septem-ber 8. Generally, afternoon photosynthetic rates (Figure 4) did not differ significantly between sites or tree sizes, except on June 15 when photosynthetic rates of trees were greater at the perennial-stream site than at the ephemeral-stream site.
Associations between leaf gas exchange and environmental or physiological variables
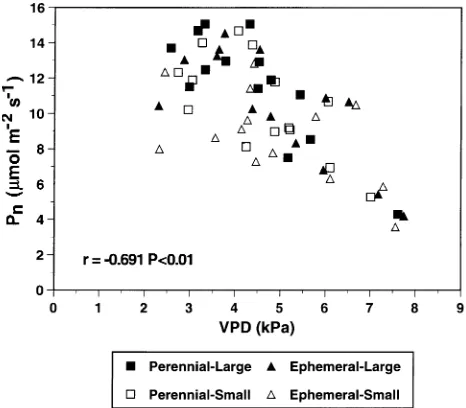
Both net photosynthetic rate and stomatal conductance were significantly and negatively correlated with vapor pressure deficit (Figure 5 and Table 3). Foliar nitrogen concentration was significantly and positively correlated only with net
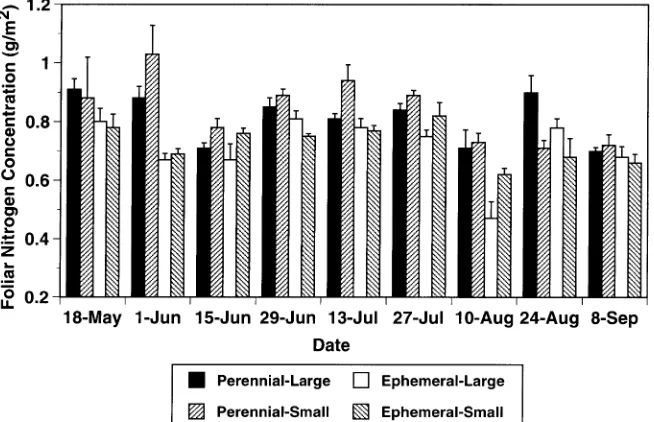
tosynthetic rate (Table 3). Site was an important source of variation for foliar nitrogen concentration. On several dates (June 1 and 29, July 13 and 27, August 10), foliar nitrogen concentration was significantly higher at the perennial-stream site than at the ephemeral-stream site (Figure 6). Foliar nitro-gen concentration differed significantly between large and small trees only on August 24 (Figure 6). Net photosynthetic rate was significantly correlated with water potential measured at the time of the gas exchange measurement, and minimum air and soil temperatures for the week preceding the gas ex-change measurement (Table 3).
Discussion
We predicted that small boxelder trees growing along the perennial stream would utilize stream water (cf. Dawson and Ehleringer 1991, Smith et al. 1991). Based on our δ18O data,
we were unable to determine directly if this prediction was correct because ground and stream water had similar δ18O values. However, differences in both predawn and mid-morn-ing water potentials between ephemeral- and perennial-stream sites were generally small and inconsistent among dates, im-plying that stream water is not a physiologically important
Figure 4. Mean mid-morning and af-ternoon net photosynthetic rates (Pn)
of boxelder trees for several dates, two stream sites (perennial, ephem-eral) and two tree size classes (large, small). Bars are equal to 1 standard error of the mean. Mid-morning net photosynthetic rate differed signifi-cantly (P ≤ 0.05) between sites on July 27 and September 8, and be-tween tree sizes on August 10 and September 8. Afternoon net photosyn-thetic rate differed significantly be-tween sites on June 15.
Figure 5. Net photosynthetic rate (Pn) versus leaf-to-air vapor pressure
water source for either large or small boxelder trees. Further, the poor correlation of variations in water potential with light-saturated net photosynthetic rate and stomatal conductance, imply that the small differences in water potential measured between sites on some dates were of little physiological
sig-nificance. In contrast to our results, stream water has been shown to be an important water source that can influence water relations and carbon gain of trees in other riparian ecosystems in western North America (Dawson and Ehleringer 1991, Smith et al. 1991).
We also expected water uptake by both small and large trees at each site to be primarily from ground- or stream water, or both, during the early summer drought, with recent precipita-tion becoming an increasingly important water source follow-ing the onset of monsoonal rains in late summer. Overall, the mixing model results support this hypothesis, although trees at the ephemeral-stream site, especially small trees, also utilized soil water in May. The source of this soil water was either winter precipitation (soil recharge), rains that occurred in late April, or both. It is unlikely that the high δ18O values of xylem water observed in early May at the ephemeral-stream site were a result of residual stem water subjected to evaporative enrich-ment throughout the previous winter (Phillips and Ehleringer 1995), because xylem water was measured only after full leaf expansion when residual water should be depleted by transpi-ration. Even on days following recent precipitation when sur-face soil water was utilized by large and small trees at both sites, this water source typically constituted no more than 40% of total water uptake.
Net photosynthetic rates differed between sites on several dates, with generally higher rates in trees at the perennial-compared with the ephemeral-stream site. Site differences in foliar nitrogen concentration seem to provide the best explana-tion of site differences in net photosynthetic rate, as foliar nitrogen concentration at the perennial-stream site was typi-cally greater than at the ephemeral-stream site. Our results showing a positive relation between foliar nitrogen concentra-tion and net photosynthetic rate reflect those reported for this species in a comparative study of male and female trees in a riparian forest in the Wasatch Mountains, Utah (Dawson and Ehleringer 1993a).
Table 3. Pearson product correlation coefficients (r) between leaf gas exchange and selected environmental and physiological variables for means pooled over measurement times, dates, sites, and tree sizes (n = 56 unless otherwise noted). (*; P = 0.05 and **; P = 0.01)
Variable Net photosynthetic Stomatal conductance rate (µmol m−2 s−1) (mol m−2 s−1)
Net photosynthetic -- +0.470** rate (µmol m−2 s−1)
Stomatal conductance +0.470** --(mol m−2 s−1)
Predawn xylem water +0.1253 +0.1213 potential (MPa) +0.2024 +0.1604
Daytime xylem water +0.361** +0.146 potential1 (MPa)
Leaf-to-air vapor --0.691** --0.270* pressure deficit (kPa)
Foliar nitrogen +0.388*3 +0.0753 concentration (g m−2)
Minimum air --0.470**3 +0.1713 temperature (°C)2
Minimum soil --0.442**3 +0.2593 temperature (°C)2
1
Measured at the time of gas exchange measurement.
2 Measured the week preceding the gas exchange measurement. 3 Mid-morning (n = 36).
4 Afternoon (n = 20).
At our field site, the most important limitation to light-satu-rated net photosynthesis and stomatal conductance of boxelder was high leaf-to-air vapor pressure deficit that developed as a result of high leaf temperatures combined with low atmos-pheric humidity. The maximum temperature of sunlit leaves in our study (approximately 40 °C) was greater than that reported for this species in a riparian forest in the midwestern United States (30--35 °C, Foster 1992), and for Salix and Populus species growing in riparian forests in intermountain basins of the Rocky Mountains (25--30 °C, Foster and Smith 1991). Based on these regional differences in leaf temperature and associated vapor pressure deficit for riparian trees, we specu-late that limitations on net photosynthesis by high vapor pres-sure deficit are stronger for riparian trees in the American Southwest than for riparian trees in cooler regions. For exam-ple, Foster (1992) reported no limitation of leaf gas exchange by vapor pressure deficit for boxelder in a riparian forest in the midwestern United States.
The significant relationship between daytime water poten-tial and net photosynthetic rate was likely the result of a strong negative correlation between water potential and vapor pres-sure deficit (r = --0.706, n = 56, P < 0.01). Lack of a strong correlation between stomatal conductance and daytime water potential further implies that net photosynthesis was not seri-ously limited by stomatal closure induced by low water poten-tial (cf. Foster 1992). Significant negative relationships between net photosynthetic rate and minimim soil and air temperatures observed the week preceding gas exchange measurements likely resulted from limitation of photosynthe-sis by high vapor pressure deficits during warm periods, and not from an effect of these variables on gas exchange. In our study, low night temperatures did not strongly limit leaf gas exchange, as has been reported for high-elevation riparian trees and shrubs in more northern locations (Young et al. 1985, Foster and Smith 1991).
Based on previous studies of size-related differences in physiology for riparian trees in western North America (Dono-van and Ehleringer 1991, Smith et al. 1991, Dawson and Ehleringer 1993a), we also expected greater water stress and lower net photosynthetic rates for small trees than for large trees. Contrary to this hypothesis, we found that, on most dates, both large and small trees had similar water potentials, net photosynthetic rates and other physiological characteristics. At the study sites, all small trees appeared to be sprouts that originated from larger trees following flood damage. These sprouts apparently have relatively large, deep root systems capable of tapping the same deep water sources as larger trees. In summary, we hypothesized a tight coupling of net carbon gain and water stress severity to stream water availability for boxelder in a montane-riparian forest in northern Arizona. The data did not support this hypothesis because the trees appeared to rely on deep water sources regardless of stream water availability. Similar leaf water potentials and gas exchange rates were recorded for both large and small trees, because small trees were vegetative sprouts from older stems that tapped the same deep water sources as large trees. Following recent precipitation, surface soil water was also utilized by
trees, especially small trees, indicating flexibility in water source use.
Acknowledgments
This research was supported by USDA Competitive Grant No. 93-37106-9485 and by a grant from the Northern Arizona University School of Forestry Mission Research Program. The authors acknow-ledge the efforts of Karin L. Doerr in field and laboratory measure-ments, A. Hope Jahren for assistance in water extraction, and Gale Eaton for conducting the mass spectrometer analyses.
References
Brown, D.E. 1982. Biotic communities of the American Southwest----United States and Mexico. Desert Plants 4, Univ. Arizona Press, Tucson, AZ, 342 p.
Busch, D.E., N.L. Ingraham and S.D. Smith. 1992. Water uptake in woody riparian phreatophytes of the southwestern United States: A stable isotope study. Ecol. Appl. 2:450--459.
Busch, D.E. and S.D. Smith. 1993. Effects of fire on water and salinity relations of riparian woody taxa. Oecologia 94:186--194. Busch, D.E. and S.D. Smith. 1995. Mechanisms associated with
de-cline of woody species in riparian ecosystems of the southwestern United States. Ecol. Monogr. 65:347--370.
Dawson, T.E. 1993. Water sources of plants as determined from xylem-water isotopic composition: perspectives on plant competi-tion, distribucompeti-tion, and water relations. In Stable Isotopes and Plant Carbon--Water Relations. Eds. J.R. Ehleringer, A.E. Hall and G.D. Farquhar. Academic Press, San Diego, CA, pp 465--496. Dawson, T.E. and J.R. Ehleringer. 1991. Streamside trees that do not
use streamside water. Nature 350:335--337.
Dawson, T.E. and J.R. Ehleringer. 1993a. Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder,
Acer negundo. Ecology 74:798--815.
Dawson, T.E. and J.R. Ehleringer. 1993b. Isotopic enrichment of water in the ‘‘woody’’ tissues of plants: Implications for plant water source, water uptake, and other studies which use the stable isotopic composition of cellulose. Geochim. Cosmochim. Acta 57:3487--3492.
Dina, S.J. and L.G. Klikoff. 1973. Carbon dioxide exchange by several streamside and scrub community species of Red Butte Canyon, Utah. Am. Midl. Nat. 89:70--80.
Donovan, L.A. and J.R. Ehleringer. 1991. Ecophysiological differ-ences among juvenile and reproductive plants of several woody species. Oecologia 86:594--597.
Ehleringer, J.R. and C.B. Osmond. 1989. Stable isotopes. In Plant Physiological Ecology. Field Methods and Instrumentation. Eds. R.W. Pearcy, J.R. Ehleringer, H.A. Mooney and P.W. Rundel. Chap-man Hall, London, pp 281--300.
Epstein, S. and T. Mayeda. 1953. Variations in the 18O content of waters from natural sources. Geochim. Cosmochim. Acta 4:213--224. Foster, J.R. 1992. Photosynthesis and water relations of the floodplain
tree, boxelder (Acer negundo L.). Tree Physiol. 11:133--149. Foster, J.R. and W.K. Smith. 1991. Stomatal conductance patterns and
environment in high elevation phreatophytes of Wyoming. Can. J. Bot. 69:647--655.
Gray, A.N. and T.A. Spies. 1995. Water content measurement in forest soils and decayed wood using time domain reflectometry. Can. J. For. Res. 25:376--385.
Issac, R.A. and W.C. Johnson. 1976. Determination of total nitrogen in plant tissue, using a block digestor. J. Assoc. Off. Anal. Chem. 59:98--100.
Johnson, R.R. and L.T. Haigt. 1984. Riparian problems and initiatives in the American Southwest: a regional prespective. In California Riparian Systems: Ecology, Conservation and Productive Manage-ment. Eds. R. Warner and C. Henricks. Univ. California Press, Berkeley, CA, pp 404--412.
Lowe, C.H. and D.E. Brown. 1982. Introduction. In Biotic Communi-ties of the American Southwest----United States and Mexico. Ed. D.E. Brown. Desert Plants 4, Univ. Arizona Press, Tucson, AZ, pp 8--16.
Meng, F.R. and P.A. Arp. 1993. Net photosynthesis and stomatal conductance of red spruce twigs before and after twig detachment. Can. J. For. Res. 23:716--721.
Phillips, S.L. and J.R. Ehleringer. 1995. Limited uptake of summer precipitation by bigtooth maple (Acer grandidentatum Nutt) and Gambel’s oak (Quercus gambelii Nutt). Trees 9:214--219. QuikChem Systems. 1992. QuikChem Method No. 13-107-06-2-D.
Lachat Instruments, Milwaukee, WI.
Ritchie, G.A. and T.M. Hinckley. 1975. The pressure chamber as an instrument for ecological research. Adv. Ecol. Res. 9:165--254. Schubert, G.H. 1974. Silviculture of southwestern ponderosa pine.
USDA For. Ser. Res. Pap. RM-123, 71 p.
Smith, S.D., A.B. Wellington, J.L. Nachlinger and C.A. Fox. 1991. Functional responses of riparian vegetation to streamflow diversion in the eastern Sierra Nevada. Ecol. Appl. 1:89--97.
Soil Survey Staff. 1990. Keys to soil taxonomy, 4th Edn. SMSS Technical Monograph No. 6, Blacksburg, Virginia, 422 p. Sternberg L.S.L., M.J. DeNiro and H.B. Johnson. 1986. Oxygen and
hydrogen isotope ratios of water from photosynthetic tissues of CAM and C3 plants. Plant Physiol. 82:428--431.
Stromberg, J.C. and D.T. Patten. 1990. Riparian vegetation instream flow requirements: A case study from a diverted stream in the eastern Sierra Nevada, California, USA. Environ. Manage. 14:185--194.
Tyree, M.T., K.J. Kolb, S.B. Rood and S. Patino. 1994. Vulnerability to drought-induced cavitation of riparian cottonwoods in Alberta--a possible factor in the decline of the ecosystem. Tree Physiol. 14:455--466.
Tyree, M.T. and T.R. Wilmot. 1990. Errors in the calculation of evaporation and leaf conductance in steady-state porometry: The importance of accurate measurement of leaf temperature. Can. J. For. Res. 20:1031--1035.
White, J.W.C. 1989. Stable isotope ratios in plants: a review of the current theory and some potential applications. In Stable Isotopes in Ecological Research. Eds. P.W. Rundel, J.R. Ehleringer and K.A. Nagy. Springer-Verlag, Berlin, pp 142--162.