Summary We hypothesized that photoinhibition of shade-developed leaves of deciduous hardwood saplings would limit their ability to acclimate photosynthetically to increased irra-diance, and we predicted that shade-tolerant sugar maple (Acer saccharum Marsh.) would be more susceptible to photoinhibi-tion than intermediately shade-tolerant red oak (Quercus ru-bra L.). After four weeks in a canopy gap, photosynthetic rates of shade-developed leaves of both species had increased in response to the increase in irradiance, although final acclima-tion was more complete in red oak. However, photoinhibiacclima-tion occurred in both species, as indicated by short-term reductions in maximum rates of net photosynthesis and the quantum yield of oxygen evolution, and longer-term reductions in the effi-ciency of excitation energy capture by open photosystem II (PSII) reaction centers (dark-adapted Fv/Fm) and the quantum
yield of PSII in the light (φPSII). The magnitude and duration of
this decrease were greater in sugar maple than in red oak, suggesting greater susceptibility to photoinhibition in sugar maple. Photoinhibition may have resulted from photodamage, but it may also have involved sustained rates of photoprotective energy dissipation (especially in red oak). Photosynthetic ac-climation also appeared to be linked to an ability to increase leaf nitrogen content. Limited photosynthetic acclimation in shade-developed sugar maple leaves may reflect a trade-off between shade-tolerance and rapid acclimation to a canopy gap.
Keywords: Acer saccharum, carbon gain, chlorophyll fluores-cence, gap, irradiance, leaf absorptance, leaf nitrogen content, photoinhibition, Quercus rubra, red oak, sugar maple.
Introduction
Saplings of many species require one or more canopy gaps, formed by treefall or branchfall, to achieve a position in the canopy and reproduce (Canham 1985, Runkle 1985, 1989). However, gap formation represents a potentially stressful event to understory saplings. Irradiances in the understory of temper-ate forests, which typically are less than 2% of irradiance incident on the canopy, can dramatically increase when a gap is formed (Canham et al. 1990), and the resulting greater input of radiation can cause substantial increases in leaf temperature (Bazzaz and Pickett 1980). Within the highly competitive
environment of a recently formed gap, the ability to acclimate to increased irradiance is advantageous. We define acclimation as the process by which physiological or morphological changes increase the capacity for carbon gain in the new environmental regime. In a mixed mesophytic temperate for-est, gap formation is most common in June, July and August (Romme and Martin 1982), thus exposing mature, shade-de-veloped foliage to a dramatic change in environment. For shade-tolerant saplings that primarily rely on one cohort of foliage for their annual carbon gain, the response to this change is important, yet poorly understood.
When plants are transferred from low to high irradiance, expanding leaves (Pearce and Lee 1969, Jurik et al. 1979, Besford 1986, Sims and Pearcy 1992) and leaves produced in the new environment (Langenheim et al. 1984, Kamaluddin and Grace 1992, Mulkey and Pearcy 1992) acclimate to in-creased irradiance; however, fully shade-developed leaves ex-posed to high irradiance may undergo a period of photoinhibition (e.g., reviews by Powles 1984, Anderson and Osmond 1987, Long et al. 1994, Osmond 1994, Pearcy 1994). Photoinhibition is a decrease in photosynthetic rate induced by visible light and represents an integration of photodamage, repair processes, and down-regulation of photosynthesis be-cause of various protective mechanisms (Krause 1988, Dem-mig-Adams 1990, Baker and Ort 1992, Öquist et al. 1992, Krause 1994, Long et al. 1994, Osmond 1994, Adams et al. 1995a).
With prolonged exposure to high irradiance (two or more weeks), the duration of photoinhibition varies considerably, and in some cases shade-grown plants remain chronically photoinhibited (Greer and Liang 1992). In many species, pho-toinhibited leaves recover to initial photosynthetic rates (Sy-vertsen 1984, Ferrar and Osmond 1986, Sims and Pearcy 1991, Nunes et al. 1993, Turnbull et al. 1993), or even increase their rates beyond the initial values (Ferrar and Osmond 1986, Bauer and Thöni 1988, Kamaluddin and Grace 1992, Turnbull et al. 1993, Lovelock et al. 1994). In other species, photosyn-thetic rates of shade-developed leaves increase after transfer to high light with no observed period of photoinhibition (Pearce and Lee 1969, Gauhl 1976, Chow and Anderson 1987, Sebaa et al. 1987, Sims and Pearcy 1992). In addition to greater susceptibility to photoinhibition of shade-developed leaves, shade-tolerant plants in general appear more susceptible to
Acclimation of shade-developed leaves on saplings exposed to
late-season canopy gaps
SHAWNA L. NAIDU and EVAN H. D
ELUCIA
Department of Plant Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA
Received July 29, 1996
photoinhibition than plants that normally grow in high-light environments (e.g., Björkman 1981, Anderson and Osmond 1987, Johnson et al. 1993, Demmig-Adams and Adams 1994). Thus, depending on the species and the developmental stage of foliage, photoinhibition may restrict the capacity to acclimate to a new growth environment.
We used complementary methods of measuring photosyn-thesis (O2 evolution and chlorophyll fluorescence), to
deter-mine the influence of photoinhibition on the acclimation response to a sudden increase in irradiance of shade-developed leaves of two temperate tree species representing different acclimation potentials. Previous results (Naidu 1996) sug-gested that mature shade-developed leaves of red oak (Quercus rubra L.) can acclimate to a late-season canopy gap more completely than those of sugar maple (Acer saccharum Marsh.). We hypothesized that photoinhibition in shade-devel-oped leaves of deciduous hardwood saplings limits photosyn-thetic acclimation and predicted that sugar maple, a shade-tolerant species, would exhibit a greater magnitude and duration of photoinhibition in response to late-season canopy gap formation than red oak, which exhibits an intermediate tolerance to shade.
Materials and methods
Experimental design
Eight-cm-tall nursery stock (Cold Stream Farm, Free Soil, MI) of sugar maple and red oak was obtained in late March and stored with moist roots in a darkened cold-room (< 5 °C) until planting (May 27). Saplings were approximately two years old and had previously been grown in 80% shade before lifting in late spring. For each species, 150 saplings were potted in soil/peat/pearlite (1/1/1 v/v) in 23 × 38-cm (15.8 liter) pots and moved to Trelease Woods, a University of Illinois ecological research area 8 km northeast of Urbana, IL. One-hundred saplings of each species were placed in deep shade in the forest understory, and the remaining plants were placed in a nearby, naturally occurring canopy gap (75 m2). Saplings were
fertil-ized one week after planting with 250 ml per pot of 0.257 g l−1
N,P,K (20,20,20) and again three weeks after planting with a top-dressing of 30 g per pot of slow-release fertilizer (Osmo-cote, Sierra Chemical Co., Milpitas, CA). Pots were mulched with peat moss to reduce evaporation from the soil and were kept well watered throughout the season by natural rainfall. Twice during the growing season, plants were sprayed with insecticide to reduce herbivory (Orthene, 327 ppm). Destruc-tion by mammals was prevented by surrounding the plots with electric fencing.
Leaf expansion for these species in the understory was complete in 20--24 days (Naidu 1996). After an additional five weeks (to ensure complete leaf development within the shaded understory), half of the understory plants were moved to the canopy gap 58 days (Transfer, Day 0) after planting. This move simulated the occurrence of a late-season (relative to leaf development) canopy gap. The experimental design consisted of three treatments, fully shade-grown plants (Shade), fully
gap-grown plants (Gap), and shade-grown plants moved to the canopy gap (Shade-Gap).
Diurnal measurements of environmental conditions were made periodically during the summer in the Shade and Gap treatments and in full sun (outside the forest). Air, leaf, and soil temperatures were measured with copper-constantan thermo-couples; and irradiance (photosynthetic photon flux density, PPFD, 400--700 nm) was measured with an LI-185B quantum sensor (Li-Cor, Inc., Lincoln, NE). Voltage or current outputs were recorded with a data logger (21X, Campbell Scientific, Logan, UT). Integrated daily irradiance in full sun was typi-cally 37 mol m−2, and was typically 4 and 40% of full sun in
the understory and gap, respectively. Maximum air, leaf and soil temperatures in full sun were typically 31, 37, and 29 °C, respectively, and temperatures in the Gap were typically 1--2 °C lower than in full sun. Maximum air and soil temperatures in the shade were typically 4--5 °C lower than in full sun; how-ever, midday leaf temperatures in the shade were as much as 10 °C lower than in the full sun. Therefore, the move from shade to gap conditions represented a 10-fold increase in irradiance and an increase in midday leaf temperatures of as much as 8--9 °C.
Gap-control plants were sampled two days before Transfer (Day −2), and again on Day 31 after Transfer. Shade-control plants were sampled immediately before Transfer (Day 0) and on Day 33 after Transfer. Shade-Gap plants were sampled on Day 2, 4, 7, 14, 21, and 28 (29 for oak) after Transfer. The transfer date was offset by one day for the two species to allow for adequate sampling on each day. In the evening before the day on which plants were to be measured, five plants of the designated treatment and species were arbitrarily selected and placed in a growth chamber with environmental conditions similar to the Shade or Gap area from which they came. The following day, photosynthetic rates were measured on one 10-cm2 leaf disc from each plant, and fluorescence was
meas-ured on an adjacent leaf, excised at the petiole. Samples were taken between 0730 and 1430 h (CST). Only leaves of similar age that had fully developed before Transfer were sampled.
Photosynthetic O2 evolution
Both CO2-saturated (5% CO2 in hydrated air) net
photosyn-thetic rates (A) and dark respiration rates (RD) were measured
with a leaf-disc O2 electrode (LD2/2, Hansatech Ltd., Norfolk,
England) according to Delieu and Walker (1981). Light-re-sponse curves were generated by measuring O2 evolution at
different irradiances provided by passing light from a fixed-output metal halogen lamp (LS2, Hansatech Ltd.) through combinations of neutral-density filters (Melles-Griot, Irvine, CA). Irradiance was measured with a Li-Cor LI-185B quantum sensor. Temperature in the O2 electrode chamber was
main-tained at 25 °C by a circulating refrigerated water bath. Leaf discs were placed in the darkened O2 electrode chamber
and RD measured when the rate became constant (10--15 min);
a Li-Cor LI-185B quantum sensor and a Taylor-type integrat-ing sphere (LI-1800-12, Li-Cor, Inc.). Leaf absorptance was calculated as 1 −R−T. Oxygen evolution rates are reported on an absorbed-irradiance basis. The quantum yield of O2
evolu-tion (φO2) was calculated as the slope of the initial linear region of the light-response curve (irradiances < 100 µmol m−2 s−1,
excluding zero). Four points on the light-response curves were located in this region.
Leaf composition
The leaf discs used for O2 electrode measurements were
oven-dried (70 °C) to a constant mass and weighed to determine leaf mass per unit area (LMA). Total Kjeldahl nitrogen was then determined with an autoanalyzer (Traacs 800, Bran and Leubbe, Buffalo Grove, IL) following acid digestion (Lowther 1980). Leaf thickness was measured at 10 interveinal positions with a dial-gauge micrometer (Starrett, Athol, MA) on the same leaves used for fluorescence measurements.
Chlorophyll a fluorescence
Chlorophyll a fluorescence was measured with a pulse-ampli-tude modulated fluorimeter (PAM-101, Walz, Effeltrich, Ger-many) on leaves adjacent to those used for O2 evolution
measurements. Detached leaves were placed on a moist pad in a light-tight chamber for dark-acclimation (30 min) before measurement. The chamber was maintained at 25 °C and flushed with hydrated 5% CO2 in air throughout the
measure-ments to overcome potential stomatal limitations caused by stomatal closure. Following dark acclimation, initial fluores-cence intensity (Fo) was measured at the leaf surface under a
low irradiance (0.06 µmol m−2 s−1) modulated measuring
beam. A 1-s pulse of saturating irradiance (12000 µmol m−2 s−1),
sufficient to close all open (oxidized) photosystem II (PSII) reaction centers, was delivered from an FL 103 saturation pulse lamp by means of a PAM-103 control unit and repeated every minute thereafter. Maximal fluorescence (Fm) was
re-corded after the first pulse, and dark-adapted variable fluores-cence (Fv=Fm−Fo) over maximal fluorescence (Fv/Fm) was
calculated. Dark-adapted Fv/Fm quantifies the efficiency of
photon capture by open PSII reaction centers (Butler and Kitajima 1975). Fluorescence nomenclature follows van Kooten and Snel (1990).
Immediately following the Fm flash, the actinic lamp (1000
µmol m−2 s−1 incident irradiance) was switched on (PAM 102). This light intensity was 95% saturating (or greater) but not photoinhibiting for these species in the Gap and Shade treat-ments (Naidu 1996). Maximal fluorescence (induced by the saturating pulses, Fm′) and steady-state fluorescence
(fluores-cence intensity between saturating pulses, Fs) during actinic
illumination were recorded after 1--20 min, by which time both were constant. After this time, the actinic and pulse lamps were switched off and minimal fluorescence (Fo′) recorded. These
data were used to calculate photochemical (qP) and
nonphoto-chemical (qN) quenching as described by Schreiber et al.
(1986) and the quantum yield of photosystem II during illumi-nation (φPSII) was calculated as described by Genty et al.
(1989). Photochemical quenching provides an approximate
measure of the number of open PSII centers and qN includes
all other potential forms of fluorescence quenching, resulting in nonradiative energy dissipation; i.e., down-regulation of photosynthesis (e.g., Baker and Ort 1992). Such processes include the ∆pH- and xanthophyll-dependent dissipation of excess energy as heat within the antenna complex (e.g., Gil-more and Yamamoto 1993, GilGil-more and Björkman 1995, Demmig-Adams and Adams 1996), and are generally assumed to protect against damage (e.g., reviews by Krause 1988, Baker and Ort 1992, Dau 1994, Osmond 1994, Demmig-Adams and Adams 1996). The quantum yield of PSII was determined by the number of open PSII centers and the excitation energy capture efficiency of those PSII reaction centers (Genty et al. 1989).
Statistical analysis
To determine the magnitude of physiological acclimation in Shade-Gap leaves, pairwise comparisons were made between Shade-Gap and Shade leaves and between Shade-Gap and Gap leaves for values measured at the end of the experiment only. For simplicity, comparisons between Shade and Gap controls are not discussed. Data were analyzed with SAS statistical software (ver. 6.10, SAS Institute Inc., Cary, NC). Because of the small sample size, pairwise comparisons were made with a Mann-Whitney U (rank sum) test, and significant differences are reported at the P < 0.05 level.
Results
Photosynthetic O2 evolution
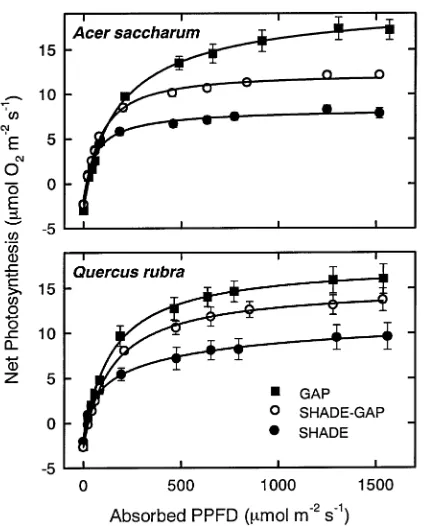
By the end of the four-week acclimation period, Shade-Gap leaves of sugar maple and red oak had greater photosynthetic rates than Shade leaves, and this increase in net photosynthesis (measured as O2 evolution) was evident over a range of
irradi-ances (Figure 1). For both species, the light-saturated photo-synthetic rate (Amax; measured at 1200--1600 µmol m−2 s−1,
PPFD) and the rate measured under sub-saturating irradiance (A200; 200 µmol m−2 s−1, PPFD) for Shade-Gap leaves
ex-ceeded values for Shade controls (P < 0.05, except P < 0.08 for Amax of red oak) by the end of the experiment (Figure 2). For
red oak, these values were not significantly different from Gap controls (P > 0.05). Leaf absorptance of Shade-Gap leaves decreased over the acclimation period but recovered to control values (Shade-Gap = Shade, P > 0.05) by the end of the experiment (Figure 3). The confounding effects of this change in leaf absorptance on photosynthesis were eliminated by calculating photosynthetic rates on an absorbed irradiance basis.
To examine the kinetics of photosynthetic acclimation, light-response curves were generated throughout the experi-ment for Shade-Gap leaves and compared to Shade and Gap controls measured before and after the four-week acclimation period. Three representative points on the light-response curve (RD, A200, and Amax) are plotted over the course of the
experi-ment in Figure 2. Shade-Gap leaves increased rates of RD after
Shade-Gap leaves of red-oak had RD rates equal to Gap leaves
(P > 0.05); however, Shade-Gap leaves of sugar maple had rates equal to Shade leaves (P > 0.05; Figure 2). In shade-de-veloped leaves, A200 and Amax decreased on exposure to the
canopy gap (Figure 2) but exceeded the Shade controls by the end of the experiment (see above). The lowest rates of net photosynthesis occurred on Day 4 with recovery to initial values by Day 7 (except A200, sugar maple). It is unclear why
the quantum yield of O2 evolution (φO2) for control plants was lower than values reported for a variety of other species (Björk-man and Demmig 1987); however, φO2 was similar between species and treatments at the beginning of the experiment and remained relatively constant across the season in control plants of sugar maple, although final values for Shade and Gap controls of red oak diverged. On exposure to the canopy gap, quantum yield was reduced in shade-developed leaves of sugar maple (Figure 4), with the maximum reduction occurring on Day 7 followed by an increase thereafter. The initial pattern is less clear in red oak, but by Day 7 φO2 had begun to increase. By the end of the experiment, φO2 of Shade-Gap leaves was greater (P < 0.05) than that of Shade leaves and did not differ significantly from that of Gap leaves (P > 0.05) for both species.
Leaf composition
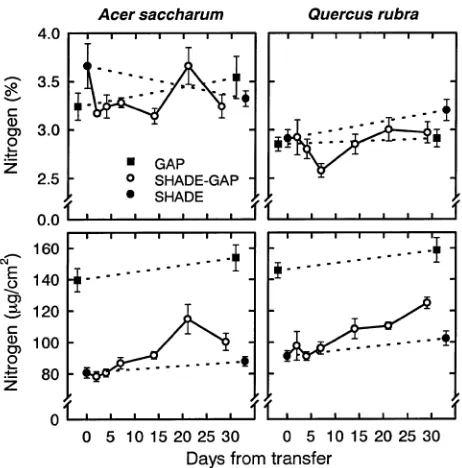
The LMA of Shade-Gap leaves increased slightly over the duration of the experiment and final values were greater than those of Shade leaves (P < 0.05) but less than those of Gap leaves (P < 0.05; Figure 5). The increase in LMA was not the result of an increase in leaf thickness, because leaf thickness decreased over the duration of the experiment in all treatments (Figure 5). In shade-developed leaves, leaf nitrogen concentra-tion calculated on a dry mass basis (%N) decreased on expo-sure to the canopy gap, but recovered to initial values by Day 21 in sugar maple and Day 14 in red oak (Figure 6). For both species, final values were not significantly different from those of Shade or Gap controls (P > 0.05). However, when calculated on an area basis, leaf nitrogen content of Shade-Gap
Figure 1. Response of net photosynthesis, measured as O2 evolution,
to absorbed photosynthetic photon flux density (PPFD) of Acer sac-charum and Quercusrubra leaves on plants grown in a canopy gap (Gap) or the forest understory (Shade) or grown in the forest under-story and transferred to a canopy gap (Shade-Gap). Light-response curves shown were measured four weeks after the date of Transfer. Means are of four or five leaves, each from a different plant, per treatment and error bars of plus and minus one standard error are shown except when smaller than symbol size. A line was fit to the data using a power function as described in DeLucia et al. (1995).
Figure 2. Dark respiration (RD), net photosynthesis at 200 µmol
m−2 s−1 absorbed irradiance (A200), and maximum net photosynthesis
(Amax, highest measured point on the light response curve) of Acer
leaves remained steady initially, increasing after about a week; final values were greater than in Shade but less than in Gap leaves (P < 0.05; Figure 6).
Chlorophyll a fluorescence
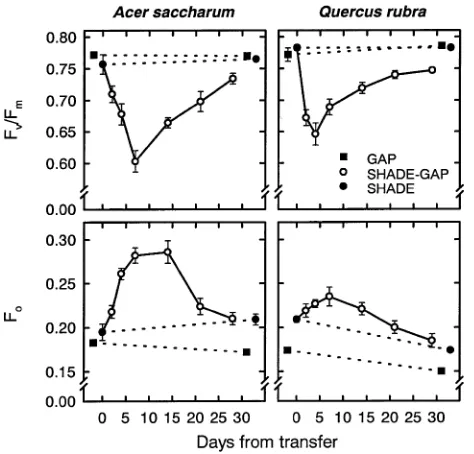
To assess the magnitude and duration of photoinhibition within shade-developed leaves on exposure to a canopy gap, fluores-cence parameters of dark-adapted leaves and quenching of fluorescence in the light were monitored throughout the ex-periment. Shadeveloped leaves exhibited an immediate de-crease in Fv/Fm on exposure to the canopy gap, with the
maximal decrease occurring by Day 7 in sugar maple and Day 4 in red oak, followed by a subsequent slow recovery (Figure 7). By the end of the experiment, Fv/Fm of Shade-Gap
leaves was still lower than that of Shade (P < 0.05) and Gap (P < 0.05, sugar maple; P < 0.08, red oak) controls. For sugar maple, the decrease in Fv/Fm was mostly a result of an increase
in Fo (reported as a percentage of the highest Fm obtained for
each species; Figure 7) because there was little change in Fm,
except on Day 14, when Fm was 5% higher than the initial
Figure 3. Absorptance of Acersaccharum and Quercusrubra leaves on plants grown in a canopy gap (Gap) or the forest understory (Shade) or grown in the forest understory and transferred to a canopy gap (Shade-Gap). Measurements, sample size, and symbols are as in Figure 2.
Figure 4. Quantum yield of O2 evolution (moles O2 evolved per mole
absorbed photons; φO2) of Acersaccharum and Quercusrubra leaves
on plants grown in a canopy gap (Gap) or the forest understory (Shade) or grown in the forest understory and transferred to a canopy gap (Shade-Gap). Measurements, sample size, and symbols are as in Figure 2.
Figure 5. Leaf mass per unit area (LMA) and interveinal leaf thickness of Acersaccharum and Quercusrubra leaves on plants grown in a canopy gap (Gap) or the forest understory (Shade) or grown in the forest understory and transferred to a canopy gap (Shade-Gap). Meas-urements, sample size, and symbols are as in Figure 2.
Figure 6. Leaf nitrogen on a dry-mass basis (%) and an area basis of
value in Shade controls (data not shown). Conversely, the decrease in Fv/Fm in red oak was mainly the result of a decrease
in Fm, which was 33% lower than the initial value in Shade
controls by Day 4, but rose to values similar to those of the Gap controls by Day 7. The rise in Fo for Shade-Gap red oak leaves
may also have contributed to the decrease in Fv/Fm. The
maxi-mum increase in Fo occurred by Day 14 for sugar maple and
Day 7 for red oak. In both species, Fo for Shade-Gap leaves
recovered by the end of the experiment (Shade-Gap = Shade, P > 0.05).
Shade-developed leaves showed a reduction in the quantum yield of photosystem II reaction centers in the light (φPSII) on
exposure to the canopy gap, with maximal reduction by Day 4 and recovery by Day 14, so that final values were not signifi-cantly different from Gap controls (P > 0.05). Shade-Gap leaves showed a small initial reduction of short duration in qP
with final values greater than those of Shade and Gap leaves (P < 0.05) in sugar maple, but equal to values in control leaves in red oak (P > 0.05; Figure 8). A sustained increase in qN from
Day 4 to Day 14 was evident for Shade-Gap leaves of sugar maple, but final values were not significantly different from Shade or Gap leaves (Figure 8). There was no difference in qN
with treatment in red oak (P > 0.05). Similar trends were seen when nonphotochemical quenching was calculated as Stern-Volmer type fluorescence quenching (Fm/Fm′ -- 1; Bilger and
Björkman 1990, data not shown).
Discussion
On exposure to higher irradiance and temperature within a canopy gap, shade-developed leaves of red oak and sugar maple underwent a period of photoinhibition (Figure 2). The reduction of net photosynthesis at sub-saturating (A200) as well
as saturating (Amax) irradiance indicates that carbon gain was
limited in these species over a range of irradiances (i.e., throughout the day, under field conditions). The actual reduc-tion in photosynthetic capacity was of short durareduc-tion, and shade-developed leaves of sugar maple and red oak began to acclimate to increased irradiance by increasing photosynthetic rates compared to shade-grown controls within two weeks of exposure to a canopy gap (Figure 2). After four weeks, accli-mation was more complete in red oak (Shade-Gap leaves had photosynthetic rates not significantly different from gap-grown controls) than in sugar maple (Figures 1 and 2), which suggests that photosynthetic rates were intrinsically limited in sugar maple, perhaps by long-term photoinhibition.
Further-Figure 7. The ratio of variable (Fv) to maximal (Fm) fluorescence
(Fv/Fm) and initial fluorescence (Fo) of dark adapted Acersaccharum
and Quercusrubra leaves on plants grown in a canopy gap (Gap) or the forest understory (Shade) or grown in the forest understory and transferred to a canopy gap (Shade-Gap). Measurements, sample size, and symbols are as in Figure 2. Each point represents an average of four or five leaves measured between 0730 and 1430 h (CST) on the same day (see Methods for details).
Figure 8. The quantum yield of photosystem II during illumination (φPSII),
photochemical quenching (qP), and nonphotochemical quenching (qN)
more, acclimation of photosynthetic CO2 uptake appears to be
correlated with an ability to increase stomatal conductance, and photosynthesis in sugar maple may be stomatally limited under natural CO2 concentrations within a canopy gap (Naidu
1996).
In contrast to red oak, Shade-Gap leaves of sugar maple, exhibited an immediate decrease in the photosynthetic effi-ciency of O2 evolution (φO2, Figure 4) in addition to the de-crease in photosynthetic capacity (Amax, Figure 2), suggesting
that a portion of photoinhibition may be the result of actual damage to some component of the photosynthetic apparatus (e.g., Osmond 1994). The rise in φO2 that occurred after Day 7 in Shade-Gap leaves of sugar maple suggests that, if present, damage is repaired or ameliorated in some way. The rise in qN
of Shade-Gap sugar maple leaves (Figure 8) over time also indicates that mechanisms for excess energy dissipation asso-ciated with photoprotection are in place for this species. The extent of potential photodamage in Shade-Gap leaves of red oak is unclear from the φO2 data alone, but if present, recovered quickly (φO2 was consistently similar to Gap controls by Day 7).
Recent studies have indicated that sustained decreases in Fm,
resulting from operation of photoprotective energy dissipation by the xanthophyll cycle, may result in artificially low calcu-lations of qN (Adams et al. 1995a, 1995b). Such a situation can
arise if qN is not fully relaxed during the dark acclimation
period preceding measurement of Fm (Gilmore and Björkman
1995). Thus, protective mechanisms may be operating in red oak as well, although they were not revealed as an increase in qN. The initial lag (Day 2, Figure 2) in the reduction of net
photosynthesis for red oak suggests that avoidance or protec-tive mechanisms are either constituprotec-tive or rapidly induced (Raven 1989), which may explain the lack of a consistent decrease in φO2. Increases in dark respiration have been re-ported in shade-developed leaves exposed to higher irradiance (Gauhl 1976, Bunce et al. 1977, Sebaa et al. 1987, Sims and Pearcy 1991, Turnbull et al. 1993, Lovelock et al. 1994), and were also seen in the present study (Figure 2). Such increases are consistent with the energetic costs of damage repair (Raven 1989) or increased production of photosynthetic machinery during the acclimation process.
The reduction in dark-adapted Fv/Fm of Shade-Gap leaves
of both species following transfer to the canopy gap reveals a decrease in the efficiency of excitation energy capture by open PSII reaction centers (Butler and Kitajima 1975). A decrease in Fv/Fm accompanied by a decrease in Fm (as in red oak),
could result from damage to PSII or from sustained, high rates of energy dissipation. Thus, although the decrease in Fv/Fm in
Shade-Gap red oak leaves may indicate photodamage, it is also consistent with the presence of sustained rates of photo-protec-tive nonphotochemical quenching.
In sugar maple (and somewhat in red oak), the decrease in Fv/Fm was coupled with an increase in Fo, the initial
fluores-cence at low irradiance arising from the bulk chlorophyll (Figure 7). A sustained increase in Fo could result from either
a reduction in the overall rate of photochemistry or a reduction in the rate of energy transfer from the pigment bed to PSII
(Butler 1978, Krause 1988). Although this may be the result of photodamage (Krause 1988, Franklin et al. 1992), true Fo is
difficult to measure and may be complicated by other proc-esses, such as fluorescence originating from photosystem I (Genty et al. 1990), reverse electron flow generated by ATP hydrolysis (Schreiber 1980, Gilmore and Yamamoto 1992a, 1992b), or nonradiative energy dissipation (Gilmore and Björkman 1995). Furthermore, it has recently been suggested that rearrangement of the antenna system (which can cause a rise in Fo) is a mechanism of photoprotection and should not
be considered damage (Ottander et al. 1995). The sustained decrease in leaf absorptance over three weeks of the acclima-tion period (Figure 3) may indicate some damage to chloro-phyll pigments causing partial bleaching. Alternatively, the reduction in absorptance may have resulted from avoidance mechanisms such as restructuring within chloroplasts or movements of chloroplasts within the mesophyll cells (see review by Raven 1989).
The quantum yield of PSII during illumination is deter-mined by both the efficiency of excitation energy capture by PSII reaction centers (i.e., Fv′/Fm′) and the number of open
(oxidized) centers (indicated by qP). The initial decrease of
φPSII in Shade-Gap leaves was caused in part by a reduction in
the number of open PSII reaction centers (initial decrease in qP, Figure 8). However the reduction in qP was minimal in red
oak, and recovered quickly in both species, with values similar to those of Gap controls by Day 14. Therefore, the slower recovery in φPSII must have been a result of the long-term
reduction in Fv′/Fm′ which was evident in both species (data
not shown). Sustained decreases were also present in dark-adapted Fv/Fm, which was the only fluorescence parameter that
did not completely recover by the end of the experiment (Figure 7). This prolonged decrease in Fv/Fm indicates
long-term photoinhibition, but by the end of the experiment, low-ered efficiency of open PSII reaction centers was no longer limiting the quantum yield of O2 evolution (Figure 4) or the
quantum yield of PSII at its actual reduction state in the light (Figure 8). There are two possible explanations for this dis-crepancy. First, the reduced efficiency may be ameliorated by increased photosynthetic capacity, indicated by an increase in the number of open PSII centers (qP). This may explain why
the recovery of φO2 (Figure 4) preceded the recovery of Fv/Fm (Figure 7) by a week or more. Also, if absorbed photons can be shunted from damaged to functional PSII centers (Raven and Samuelsson 1986), φO2 could remain high although Fv/Fm was low. Second, because a majority of emitted fluorescence comes from the first layer of palisade cells within the leaf (Bornman et al. 1991), fluorescent measurements assess pho-tosynthesis of the upper leaf surface only, whereas measure-ments of O2 evolution integrate whole-leaf photosynthetic
function. Also, photoinhibitory damage to leaves appears to be concentrated in the upper leaf surface (Powles and Björkman 1982, Krause and Somersalo 1989, Nishio et al. 1994). Greater efficiency in lower layers of the leaf may compensate for photoinhibition at the leaf surface.
fluorescence parameters (Figure 7), and quenching of fluores-cence in the light (Figure 8), were greater in sugar maple than in red oak. Although the differences between species were small, the more prolonged decrease in photosynthetic parame-ters for sugar maple suggests that longer-term photoinhibition may be limiting more complete or faster acclimation relative to red oak. By the end of the experiment, Shade-Gap leaves of red oak had completely acclimated (photosynthetic rates were not significantly different from Gap controls), whereas sugar maple had not. It is possible that the length of time necessary for physiological acclimation differs among species and that complete acclimation in sugar maple might occur after four weeks (longer than the duration of this study). However, be-cause photosynthetic rates of both sun and shade leaves of temperate deciduous trees (including sugar maple and red oak) maintain a relatively constant maximum from June through September (Jurik 1986), it is unlikely that losses in carbon gain resulting from slower acclimation can be offset by increasing rates of photosynthesis beyond the rates of the gap controls. Therefore, red oak, which acclimates more quickly than sugar maple, may have a competitive advantage over sugar maple saplings of the same initial size because of greater cumulative carbon gain within the season of gap formation. The more limited acclimation response of sugar maple may reflect a trade-off between shade-tolerance and rapid exploitation of canopy gaps.
The ability of shade-developed leaves to increase nitrogen content on exposure to higher irradiance, either through inter-nal reallocation or increased nitrogen uptake from the soil, may also influence the magnitude of photoinhibition and ca-pacity for acclimation (Castro et al. 1995, Hikosaka and Terashima 1995, Naidu 1996). Increased leaf nitrogen can result from greater protein content associated with higher photosynthetic rates (Ferrar and Osmond 1986, Field and Mooney 1986, Evans 1989) or repair of photoinhibitory dam-age (Nunes et al. 1993), and the latter may explain why nitrogen fertilization decreases plant susceptibility to photoin-hibition (Ferrar and Osmond 1986, Nunes et al. 1993). In the current study, nitrogen content per unit area of shade-devel-oped leaves increased after exposure to the canopy gap, al-though this increase began to reverse in sugar maple by the end of the experiment (Figure 6). The lack of a faster or more dramatic final increase (especially in sugar maple) of nitrogen content of Shade-Gap leaves may have limited the acclimation process. Despite the increase in leaf nitrogen per unit leaf area, %N content decreased for two weeks before recovering (Fig-ure 6). This decrease in %N probably resulted from dilution by carbohydrate accumulation associated with higher net photo-synthetic rates. This is supported by the slow increase in LMA, which was not a result of increased leaf thickness (Figure 5).
In addition to acclimation of pre-existing leaves, some spe-cies produce more than one flush of leaves within a growing season. Because newly emergent leaves tend to be acclimated to the light regime in which they develop (Boardman 1977, Björkman 1981, Anderson and Osmond 1987, Givnish 1988, Abrams and Kubiske 1990), such species have the capacity to produce new, high-light acclimated leaves on exposure to a
canopy gap. New leaves would contribute substantially to whole-plant carbon gain, yet seasonal carbon gain could be even greater if old leaves also acclimate. Shade-developed red oak saplings can produce more leaves on exposure to a gap, as can sugar maple, although to a much lesser extent (Naidu 1996). However, the production of these new, ‘‘sun-type’’ leaves (they would presumably be very similar to Gap con-trols) does not come at the expense of acclimation of pre-ex-isting leaves, which suggests that acclimation includes a variety of integrated responses from physiological to alloca-tional changes (Naidu 1996). Other whole-plant acclimation responses have been observed in red oak saplings and include increased root growth to offset higher water-demand in gaps, and carbohydrate storage in preparation for increased growth in the growing season following gap formation (Naidu 1996).
Acknowledgments
The authors are grateful to Govindjee, Adam Gilmore and Taylor Feild for insightful discussions on photoinhibition. This study was sup-ported, in part, by a McKnight Foundation Graduate Fellowship for Interdisciplinary Research in Photosynthesis; and a Graduate College Dissertation Research Grant and Francis M. and Harlie M. Clark Summer Grant (School of Life Sciences) from the University of Illinois at Urbana-Champaign to S.L.N. Additional support was pro-vided by USDA Grants #9401296 (NRICGP, Ecosystems) and #89-37280-4817 (Stratospheric Ozone Reduction Program) to E.H.D.
References
Abrams, M.D. and M.E. Kubiske. 1990. Leaf structural characteristics of 31 hardwood and conifer tree species in central Wisconsin: influence of light regime and shade-tolerance rank. For. Ecol. Man-age. 31:245--253.
Adams III, W.W., B. Demmig-Adams, A.S. Verhoeven and D.H. Barker. 1995a. ‘‘Photoinhibition’’ during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust. J. Plant Physiol. 22:261--276.
Adams III, W.W., A. Hoehn and B. Demmig-Adams. 1995b. Chilling temperatures and the xanthophyll cycle. A comparison of warm-grown and overwintering spinach. Aust. J. Plant Physiol. 22:75--85. Anderson, J.M. and C.B. Osmond. 1987. Shade-sun responses: com-promises between acclimation and photoinhibition. In Photoinhibi-tion. Eds. D.J. Kyle, C.B. Osmond and C.J. Arntzen. Elsevier Science Publishers, New York, pp 1--38.
Baker, N.R. and D.R. Ort. 1992. Light and crop photosynthetic per-formance. In Crop Photosynthesis: Spatial and Temporal Determi-nants. Eds. N.R. Baker and H. Thomas. Elsevier Science Publishers, New York, pp 289--312.
Bauer, H. and W. Thöni. 1988. Photosynthetic light acclimation in fully developed leaves of the juvenile and adult life phases of
Hederahelix. Physiol. Plant. 73:31--37.
Bazzaz, F.A. and S.T.A. Pickett. 1980. Physiological ecology of tropi-cal succession: a comparative review. Annu. Rev. Ecol. Syst. 11:287--310.
Bilger, W. and O. Björkman. 1990. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced ab-sorbance changes, fluorescence and photosynthesis in leaves of
Hedera canariensis. Photosynth. Res. 25:173--185.
Björkman, O. 1981. Responses to different quantum flux densities. In
Physiological Plant Ecology I: Responses to the Physical Environ-ment. Eds. O.L. Lange, P.S. Nobel, C.B. Osmond and H. Ziegler. Springer-Verlag, New York, pp 57--107.
Björkman, O. and B. Demmig. 1987. Photon yield of O2 evolution and
chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta 170:489--504.
Boardman, N.K. 1977. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 28:355--377.
Bornman, J.F, T.C. Vogelmann and G. Martin. 1991. Measurement of chlorophyll fluorescence within leaves using a fibreoptic micro-probe. Plant Cell Environ. 14:719--725.
Bunce, J.A., D.T. Patterson, M.M. Peet and R.S. Alberte. 1977. Light acclimation during and after leaf expansion in soybean. Plant Physiol. 60:255--258.
Butler, W.L. 1978. Energy distribution in the photochemical apparatus of photosynthesis. Annu. Rev. Plant Physiol. 29:345--378. Butler, W.L. and M. Kitajima. 1975. Fluorescence quenching in
pho-tosystem II of chloroplasts. Biochim. Biophys. Acta 376:116--125. Canham, C.D. 1985. Suppression and release during canopy
recruit-ment in Acersaccharum. Bull. Torrey Bot. Club 112:134--145. Canham, C.D., J.S. Denslow, W.J. Platt, J.R. Runkle, T.A. Spies and
P.S. White. 1990. Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Can. J. For. Res. 20:620--631.
Castro, Y., N. Fetcher and D.S. Fernández. 1995. Chronic photoinhibi-tion in seedlings of tropical trees. Physiol. Plant. 94:560--565. Chow, W.S. and J.M. Anderson. 1987. Photosynthetic responses of
Pisumsativum to an increase in irradiance during growth I. Photo-synthetic activities. Aust. J. Plant Physiol. 14:1--8.
Dau, H. 1994. Short-term adaptation of plants to changing light inten-sities and its relation to photosystem II photochemistry and fluores-cence emission. J. Photochem. Photobiol. B Biol. 26:3--27. Delieu, T.J. and A. Walker. 1981. Polarographic measurements of
photosynthetic oxygen evolution in leaf disks. New Phytol. 86:165--178.
DeLucia, E.H., K. Nelson, T.C. Vogelmann and W.K. Smith. 1995. Contribution of intercellular reflectance to photosynthesis in shade leaves. Plant Cell Environ. 19:159--170.
Demmig-Adams, B. 1990. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1020:1--24.
Demmig-Adams, B. and W.W. Adams, III. 1994. Capacity for energy dissipation in the pigment bed in leaves with different xanthophyll cycle pools. Aust. J. Plant Physiol. 21:575--588.
Demmig-Adams, B. and W.W. Adams, III. 1996. The role of xantho-phyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1:21--26.
Evans, J.R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9--19.
Ferrar, P.J. and C.B. Osmond. 1986. Nitrogen supply as a factor influencing photoinhibition and photosynthetic acclimation after transfer of shade-grown Solanumdulcamara to bright light. Planta 168:563--570.
Field, C. and H.A. Mooney. 1986. The photosynthesis--nitrogen rela-tionship in wild plants. In On the Economy of Plant Form and Function. Ed. T.J. Givnish. Cambridge University Press, Cam-bridge, pp 25--55.
Franklin, L.A., G. Levavasseur, C.B. Osmond, W.J. Henley and J. Ramus. 1992. Two components of onset and recovery during photoinhibition of Ulva rotunda. Planta 186:399--408.
Gauhl, E. 1976. Photosynthetic response to varying light intensity in ecotypes of Solanumdulcamara L. from shaded and exposed habi-tats. Oecologia 22:275--286.
Genty, B., J.-M. Briantais and N.R. Baker. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990:87--92.
Genty, B., J. Wonders and N.R. Baker. 1990. Non-photochemical quenching of Fo in leaves is emission wavelength dependent:
con-sequences for quenching analysis and its interpretation. Photosynth. Res. 26:133--139.
Gilmore, A.M. and H.Y. Yamamoto. 1992a. Dark induction of zeaxan-thin-dependent nonphotochemical fluorescence quenching medi-ated by ATP. Proc. Nat. Acad. Sci. 89:1899--1903.
Gilmore, A.M. and H.Y. Yamamoto. 1992b. Zeaxanthin-dependent quenching of the variable fluorescence arising from ATP-induced reverse electron flow. In Research in Photosynthesis. Ed. N. Mu-rata. Kluwer Academic Publishers, Netherlands, pp 255--258. Gilmore, A.M. and H.Y. Yamamoto. 1993. Biochemistry of
xantho-phyll-dependent nonradiative energy dissipation. In Photosynthetic Responses to the Environment. Eds. H.Y. Yamamoto and C.M. Smith. Am. Soc. Plant Physiologists, Beltsville, MD, pp 162--165. Gilmore, A.M. and O. Björkman. 1995. Temperature-sensitive cou-pling and uncoucou-pling of ATPase-mediated, nonradiative energy dissipation: similarities between chloroplasts and leaves. Planta 197:646--654.
Givnish, T.J. 1988. Adaptation to sun and shade: a whole-plant per-spective. In Ecology of Photosynthesis in Sun and Shade. Eds. J.R. Evans, S. von Caemmerer and W.W. Adams, III. CSIRO, Canberra, Australia, pp 63--92.
Greer, D.H. and W.A. Laing. 1992. Photoinhibition of photosynthesis in intact kiwifruit (Actinidiadeliciosa) leaves: changes in suscepti-bility to photoinhibition and recovery during the growth season. Planta 186:418--425.
Hikosaka, K. and I. Terashima. 1995. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with
respect to nitrogen use. Plant Cell Environ.18:605--618.
Johnson, G.N., A.J. Young, J.D. Scholes and P. Horton. 1993. The dissipation of excess excitation energy in British plant species. Plant Cell Environ.16:673--679.
Jurik, T.W. 1986. Seasonal patterns of leaf photosynthetic capacity in successional northern hardwood tree species. Am. J. Bot. 73:131--138.
Jurik, T.W., J.F. Chabot and B.F. Chabot. 1979. Ontogeny of photosyn-thetic performance in Fragariavirginiana under changing light regimes. Plant Physiol. 63:542--547.
Kamaluddin, M. and J. Grace. 1992. Photoinhibition and light accli-mation in seedlings of Bischofiajavanica, a tropical forest tree from Asia. Ann. Bot. 69:47--52.
Krause, G.H. 1988. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 74:566--574.
Krause, G.H. 1994. Photoinhibition induced by low temperatures. In
Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field. Eds. N.R. Baker and J.R. Bowyer. Bios Scientific Pub-lishers, Oxford, U.K., pp 331--348.
Langenheim, J.H., C.B. Osmond, A. Brooks and P.J. Ferrar. 1984. Photosynthetic responses to light in seedlings of selected Ama-zonian and Australian rainforest tree species. Oecologia 63:215--224.
Long, S.P., S. Humphries and P.G. Falkowski. 1994. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45:633--662.
Lovelock, C.E., M. Jebb and C.B. Osmond. 1994. Photoinhibition and recovery in tropical plant species: response to disturbance. Oecolo-gia 97:297--307.
Lowther, J.R. 1980. Use of single sulphuric acid--hydrogen peroxide digest for the analysis of Pinusradiata needles. Commun. Soil Sci. Plant Anal. 11:175--188.
Mulkey, S.S. and R.W. Pearcy. 1992. Interactions between acclimation and photoinhibition of photosynthesis of a tropical forest under-story herb, Alocasiamacrorrhiza (L.) G. Don, during simulated canopy gap formation. Funct. Ecol. 6:719--729.
Naidu, S.L. 1996. Coordination of physiological and morphological acclimation of shade-grown tree saplings to late-season canopy gap formation. Ph.D. Thesis, Univ. Illinois, Urbana, IL, 133 p. Nishio, J.N., J. Sun and T.C. Vogelmann. 1994. Photoinhibition and
the light environment within leaves. In Photoinhibition of Photo-synthesis: From Molecular Mechanisms to the Field. Eds. N.R. Baker and J.R. Bowyer. Bios Scientific Publishers, Oxford, U.K., pp 221--237.
Nunes, M.A., J.D.C. Ramalho and M.A. Dias. 1993. Effect of nitrogen supply on the photosynthetic performance of leaves from coffee plants exposed to bright light. J. Exp. Bot. 44:893--899.
Öquist, G., W.S. Chow and J.M. Anderson. 1992. Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 186:450--460.
Osmond, C.B. 1994. What is photoinhibition? Some insights from comparisons of shade and sun plants. In Photoinhibition of Photo-synthesis: From Molecular Mechanisms to the Field. Eds. N.R. Baker and J.R. Bowyer. Bios Scientific Publishers, Oxford, U.K., pp 1--25.
Ottander, C., D. Campbell and G. Öquist. 1995. Seasonal changes in photosystem II organisation and pigment composition in Pinus sylvestris. Planta 197:176--183.
Pearce, R.B. and D.R. Lee. 1969. Photosynthetic and morphological adaptation of alfalfa leaves to light intensity at different stages of maturity. Crop Sci. 9:791--794.
Pearcy, R.W. 1994. Photosynthetic response to sunflecks and light gaps: mechanisms and constraints. In Photoinhibition of Photosyn-thesis: From Molecular Mechanisms to the Field. Eds. N.R. Baker and J.R. Bowyer. Bios Scientific Publishers, Oxford, U.K., pp 255--271.
Powles, S.B. 1984. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 35:15--44.
Powles, S.B. and O. Björkman. 1982. Photoinhibition of photosynthe-sis: effect on chlorophyll fluorescence at 77K in intact leaves and in chloroplast membranes of Nerium oleander. Planta 156:97--107. Raven, J.A. 1989. Fight or flight: the economics of repair and
avoid-ance of photoinhibition of photosynthesis. Funct. Ecol. 3:5--19. Raven, J.A. and G. Samuelsson. 1986. Repair of photoinhibitory
damage in Anacystisnidulans 625 (Synechococcus 6301): relation to catalytic capacity for, and energy supply to, protein synthesis, and implications for µmax and the efficiency of light-limited growth.
New Phytol. 103:625--643.
Romme, W.H. and W.H. Martin. 1982. Natural disturbance by tree falls in old-growth mixed mesophytic forest: Lilley cornett woods, Kentucky. Proc. Fourth Central Hardwood Forest Conference. Univ. Kentucky, Lexington, KY, pp 367--383.
Runkle, J.R. 1985. Disturbance regimes in temperate forests. In The Ecology of Natural Disturbance and Patch Dynamics. Eds. S.T.A. Pickett and P.S. White. Academic Press, Orlando, FL, pp 17--33. Runkle, J.R. 1989. Synchrony of regeneration, gaps, and latitudinal
differences in tree species diversity. Ecology 70:546--547. Schreiber, U. 1980. Light-activated ATPase and ATP-driven reverse
electron transport in intact chloroplasts. FEBS Lett. 122:121--124. Schreiber, U., U. Schliwa and W. Bilger. 1986. Continuous recording
of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10:51--62.
Sebaa, El D., J.L. Prioul and J. Brangeon. 1987. Acclimation of adult
Loliummultiflorum leaves to changes in irradiance: effect on leaf photosynthesis and chloroplast ultrastructure. J. Plant Physiol. 127:431--441.
Sims, D.A. and R.W. Pearcy. 1991. Photosynthesis and respiration in
Alocasia macrorrhiza following transfers to high and low light. Oecologia 86:447--453.
Sims, D.A. and R.W. Pearcy. 1992. Response of leaf anatomy and photosynthetic capacity in Alocasia macrorrhiza (Araceae) to a transfer from low to high light. Am. J. Bot. 79:449--455.
Syvertsen, J.P. 1984. Light acclimation in Citrus leaves. II. CO2
assimilation and light, water, and nitrogen use efficiency. J. Am. Soc. Hortic. Sci. 109:812--817.
Turnbull, M.H., D. Doley and D.J. Yates. 1993. The dynamics of photosynthetic acclimation to changes in light quantity and quality in three Australian rainforest tree species. Oecologia 94:218--228. van Kooten, O. and F.H. Snel. 1990. The use of chlorophyll