Predation on fungal and bacterial biomass in a soddy-podzolic soil
amended with starch, wheat straw and alfalfa meal
Anvar Sh. Mamilov
a, Boris A. Byzov
b, Dmitri G. Zvyagintsev
b, Oliver M. Dilly
a,∗ aÖkologie-Zentrum, Universität Kiel, Schauenburgerstraße 112, 24118 Kiel, GermanybDepartment of Soil Biology, Faculty of Soil Science, Moscow State University, 119899 Moscow, Russia
Received 6 December 1999; received in revised form 13 July 2000; accepted 13 July 2000
Abstract
The variation in bacterial, fungal and total microbial biomass and activity was studied together with the abundance of soil nematodes and microarthropods after the addition of substrates differing in nitrogen availability to a soddy-podzolic soil. The experiments were carried out in microcosms with native and defaunated soil to evaluate stimulatory and suppressive effects of the microfauna on soil micro-organisms. Predation by microfauna (nematodes) and mesofauna (microarthropods) reduced the microbial biomass and microbial respiration by approximately 25% after addition of nitrogen rich alfalfa meal. When starch and wheat straw were supplied, the microbial biomass and activity were stimulated by up to 30% by grazing. Thus, the effect of predation on the microbiota depended on the composition of the available substrates and available nitrogen seems to be an important factor controlling stimulation or suppression of soil micro-organisms by the soil fauna when fresh organic compounds are accessible. The presence of soil fauna stimulated bacteria and, thus, reduced the fungal/bacterial ratio during the course of decomposition. In contrast, the fungal/bacterial ratio declined due to decreasing fungal biomass in defaunated soil. © 2001 Elsevier Science B.V. All rights reserved.
Keywords: Fauna; Litter decomposition; Micro-organisms; Nitrogen; Stimulation; Suppression
1. Introduction
The interaction between fauna and micro-organisms in soil is of great importance for the decomposition of natural substrates and, thus, for the biogeochemistry of ecosystems (Brussaard, 1998). The mineralisation of organic substances in soil is mainly performed by micro-organisms, but soil fauna have a modulating and stimulatory impact (Anderson et al., 1983). The rate of nitrogen and phosphorus liberation in soil is affected by the presence of soil animals (Ingham et al., 1985).
∗Corresponding author. Tel.:+49-431-880-4085;
fax:+49-431-880-4083.
E-mail address: [email protected] (O.M. Dilly).
However, these processes are regulated by the commu-nity structure and the complex nutritional interactions within the soil biota (Huhta and Setälä, 1990; Ingham et al., 1985; Setälä et al., 1990).
Several studies showed that the presence of soil animals increased the content of available nutrients, decomposition rate of organic substances, microbial growth and respiration rate (Ineson et al., 1982; Clarholm, 1985; McGonigle, 1995; Alphei et al., 1996). However, some investigations indicate that soil fauna may also suppress microbiological components and microbial growth (Larsen and Jakobsen, 1996; Groffmann, 1999). The interaction between fauna and micro-organisms seems to depend on the nutritional conditions that vary between different soil horizons
(Dilly and Munch, 1998); e.g. Kandeler et al. (1999) showed that microarthropods did not significantly af-fect substrate-induced respiration or microbial C, N and P content in the L/F layer containing fresh organic substances but promoted microbial biomass, protease activity and phosphate content in the deeper H layer. Hence, it is still unclear how microbial growth and predation interact under various environmental con-ditions.
Therefore, we studied the effect of natural sub-strates with different quality on the outcome of faunal– microbial interaction, namely microbial suppression and stimulation. The dynamics of bacterial, fungal and total microbial biomass and respiration rates in the presence of available litter components were deter-mined with regard to the abundance of the micro- and meso-fauna. Substrates having high nitrogen content were compared to those containing low or no nitrogen reserves.
2. Materials and methods
2.1. Soil
Ten soil samples of 500 g each were randomly collected around a fir-tree from the A horizon (0–15 cm) in a region with predominantly eutric Podzoluvi-sols (FAO, 1988). The subsequently named ‘soddy-podzolic soil’ was located under coniferous forest, in the Chashnikovo area, close to Moscow, and taken in 1997. After removing large roots and litter fragments,
Table 1
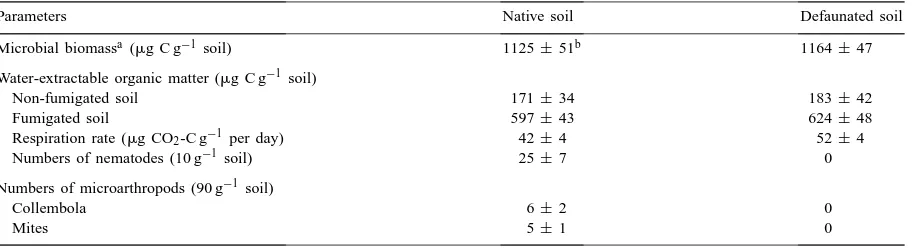
Characteristics of the native and defaunated soils as determined on day zero
Parameters Native soil Defaunated soil
Microbial biomassa(mg C g−1soil) 1125±51b 1164±47
Water-extractable organic matter (mg C g−1 soil)
Non-fumigated soil 171±34 183±42
Fumigated soil 597±43 624±48
Respiration rate (mg CO2-C g−1 per day) 42±4 52±4
Numbers of nematodes (10 g−1 soil) 25±7 0
Numbers of microarthropods (90 g−1 soil)
Collembola 6±2 0
Mites 5±1 0
aMicrobial biomass C was measured by fumigation-extraction,k
EC=2.64. bValue of the standard deviation (n=3).
the samples were thoroughly mixed and sieved at 5 mm mesh size. Soil samples were afterwards pre-incubated at natural moisture content (17% of dry mass corresponding 65% WHC) in plastic bags for 14 days at 12◦C. The soil contained 12.1 mg organic C g−1 dry soil, 0.9 mg total N g−1 dry soil, had a pH (H2O) value of 4.6 and contents of
exchange-able NH4+-N and NO3−-N of 7.2 and 5.8 mg kg−1,
respectively.
2.2. Experimental design
Glass flasks (volume 150 ml) were filled with 100 g of either native or defaunated soil. Available defauna-tion procedures are physical exclusion of the animals (small mesh bags), application of biocides, microwave treatment, deep-freezing in combination with drying and moist heat treatment. Although these procedures provide sufficient defaunation, they may cause un-desired and unpredictable changes in soil physical, chemical and microbiological characteristics such as CO2 evolution (Huhta et al., 1989; Wright et al.,
1989).
We used the method of Wright et al. (1989), which was modified as follows. The samples were placed in a plastic bag in 2–3 cm layers and then heated at 55◦C
for 5 h. Heating at 55◦C for 5 h completely eliminated
during 11 days, did not change significantly in either the defaunated or the native soil. It varied from 17.3 to 19.4 and 17.7 to 21.4mg CO2-C g−1 soil per day
in the native and defaunated soils, respectively. Our defaunation procedure did not completely kill animal eggs but recovery of the animal populations took about 14–25 days, dependent on taxa, and, thus could only have interfered with the results during the final part of our experiment.
One percent of alfalfa meal with a C/N ratio of approximately 32 (w/w), wheat straw with a C/N ratio of approximately 82 (w/w), or starch containing no N were added to each microcosm. As a control, native soil without any amendment was also applied. The experimental flasks were kept in the dark at 23◦C for 1 month.
Three replicate samples from each treatment were analysed after 3, 7, 10, 14, 18, 21 and 23 days for bacterial and fungal and total biomass and respiration rate. The nematodes were counted on days 0, 15 and 23 and microarthropods on days 0 and 23. Only one flask of each treatment was destructively analysed per sampling date. This was considered to be acceptable since the variation of respiration rate in defaunated and native soil samples in preliminary experiment did not exceed 3.5% in three independent microcosms over 11 days.
2.3. Bacterial, fungal and total biomass
Bacterial, fungal and total biomass C were esti-mated by the substrate-induced respiration technique following Anderson and Domsch (1973, 1978) and modified by West (1986). Fresh soil corresponding to 1 g of oven-dry material received (A) no inhibitor, (B) 8 mg streptomycin (Fluka), (C) 65 mg cycloheximide (Sigma), (D) streptomycin and cycloheximide, each dissolved in 1.5 ml of de-ionised water. After 3 h of incubation at 25◦C, 8 mg glucose dissolved in 0.5 ml
was added to the soil suspension to stimulate respira-tory response. Soil respiration was determined hourly. Treatment D was applied so as to completely inhibit the soil microbial communities (Mamilov et al., 2000). The applied concentrations of antibiotics were tested for the selectivity of the inhibition effect. The ratio((A−B)+(A−C))A−1was initially adjusted to 1±0.05.
Total microbial biomass C in the treatments with starch and alfalfa meal was calculated according to West and Sparling (1986) using the equation, micro-bial C (mg C g−1 soil) = 433 log10A (ml CO2g−1
soil per hour)+59.2. The ratio of fungal to bacterial biomass was calculated from the equation (A−B)(A−
C)−1. Fungal and bacterial C was calculated on the base of its contribution to total (non-inhibited) micro-bial respiratory response.
Microbial biomass C in the treatments with wheat straw was estimated using fumigation-extraction ac-cording to Vance et al. (1987) with a soil-extractant ratio of 1:4 (dry soil equivalent: 0.5 M potassium sulphate solution) and a kECfactor of 0.33 (Sparling
and West, 1988). Organic C in 1.6 ml extract was digested with 2.4 ml 66.7 mM K2Cr2O7 dissolved in
15.6 M H2SO4. Absorbance was determined
photo-metrically at 590 nm. Corresponding biomass val-ues were estimated either by fumigation-extraction with a conversion factor of 0.33 after Sparling and West (1988), or by substrate-induced respiration with the conversation formula after West and Sparling (1986) as used in our experiments (Mamilov et al., 2000).
All respiration measurements (CO2), including
basal respiration, were quantified by gas chromatog-raphy. The carrier gas was He with a flow rate of approximately 30 ml per minute, and the injector, column and detector temperatures were 50, 35 and 140◦C, respectively.
2.4. Animal extraction
Nematodes were extracted using Baermann funnels from samples containing 10 g of fresh soil. Micro-arthropods were extracted over four days in Tull-gren funnels containing 90 g of the soil. In the extracts total numbers of nematodes, Collembola and mites were counted under a light microscope (Cairns, 1960).
2.5. Statistics
3. Results and discussion
3.1. Bacterial, fungal and microbial biomass content and respiratory activity
The application of both alfalfa meal and starch led to the macroscopically visible development of mycelium on the soil surface of all microcosms in the presence of the fauna. In defaunated soil, the mycelium was only visible in the soil treated with alfalfa meal but not with starch. This indicates that soil fauna apparently
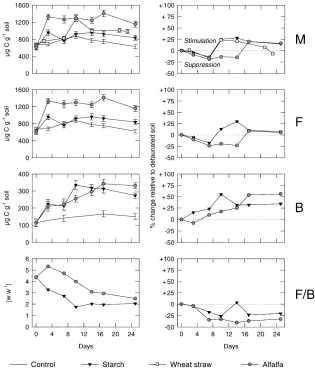
Fig. 1. Microbial (M), fungal (F) and bacterial (B) biomass and fungal/bacterial ratio (F/B) in native soil (left side), and the effect of fauna on microbiological characteristics compared to defaunated soil (right side) during the course of decomposition of starch, wheat straw and alfalfa meal; bars indicate standard deviations.
stimulated fungal growth when substrate containing no nitrogen was added.
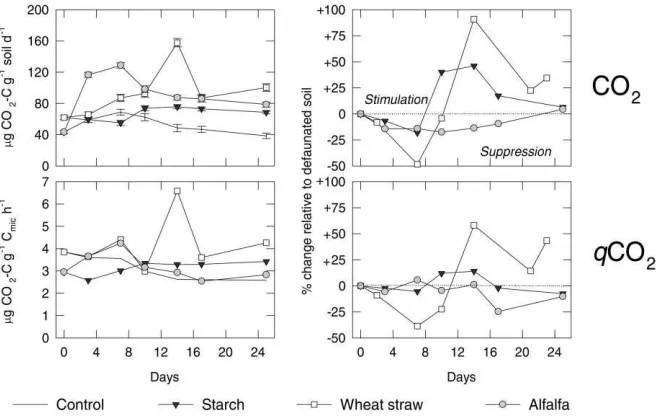
Fig. 2. Microbial respiration rates CO2 and metabolic quotient qCO2in native soil (left side), and the effect of fauna on microbiological characteristics compared to defaunated soil (right side) during the course of decomposition of starch, wheat straw and alfalfa meal; bars indicate standard deviations.
rates in the native soil (Fig. 2) exceeded those values of the defaunated soil by approximately 20–30% after 10 days. In contrast, soil amended with N-rich alfalfa meal showed an approximately 25% higher level of fungal biomass in the absence of animals. Throughout the whole experiment, the rate of CO2evolution was
also higher in the defaunated treatments (Fig. 2). At the end of the experiment, the bacterial biomass was approximately 40% higher in the presence of soil nematodes and microarthropods when soil was amended with starch and alfalfa meal (Fig. 1). No sig-nificant changes in bacterial biomass occurred in the absence of soil fauna after three days of incubation (data not shown).
In the treatments with wheat straw, the microbial biomass was lower in native than in defaunated soil until day 7 (Fig. 1). After day 10, the presence of nematodes and microarthropods permitted microbial biomass values of up to 1300mg C g−1 soil, which was approximately 25% higher than in the defaunated soil. A similar, more pronounced, trend was found for the respiration rate (Fig. 2). The respiration rate was higher in the defaunated soil in the first week of incubation. The effect of straw addition resembled more that of starch than that of alfalfa meal.
3.2. Dynamics of fungal-to-bacterial ratio
The ratio of fungal to bacterial biomass decreased in all treatments independently of the substrate composi-tion during the course of the experiment (Fig. 1). In the presence of soil animals, the fungal/bacterial ratio de-creased from 5.3 and 5.6 to 2.0 and 2.5 for treatments with starch and alfalfa meal, respectively. This was mainly due to bacterial growth. Considering that the presence of fauna support N liberation from consumed microbial cells, our findings agree with observations of Bardgett and McAlister (1999) who showed that high N availability in grassland soils favoured bacteria more than fungi and, thus, decreased fungal/bacterial ratio. In defaunated soil, the fungal biomass de-clined with no significant changes in bacterial biomass.
3.3. Faunal abundance
Table 2
Abundance of soil micro- and mesofauna during decomposition of alfalfa meal and starch in a soddy-podzolic soil
Treatment Day
0 16 25
Nematodes (number per 10 g−1 soil)
Native+alfalfa meal 280±38a 1816±47 2564±62
Defaunated+alfalfa meal 0 8±1 254±44
Native+starch 280±38 427±52 726±57
Defaunated+starch 0 5±1 72±12
Microarthropods (number per 90 g−1 soil)
Native+alfalfa meal 32±4 NDb 52±7
Defaunated+alfalfa meal 0 ND 0
Native+starch 32±4 ND 61±5
Defaunated+starch 0 ND 0
aStandard deviation. bND: not determined.
numbers of nematodes were more enhanced than those of soil mites and Collembola. Thus, the varia-tion of nematode biomass appears more interrelated to microbial biomass. Grazing of microarthropods may certainly affect microbial biomass and respiration ac-tivity but probably mainly in decomposing faeces of macrofauna (Van der Drift and Jansen, 1977) or fresh leaf litter (Hanlon and Anderson, 1979; Ineson et al.,
Table 3
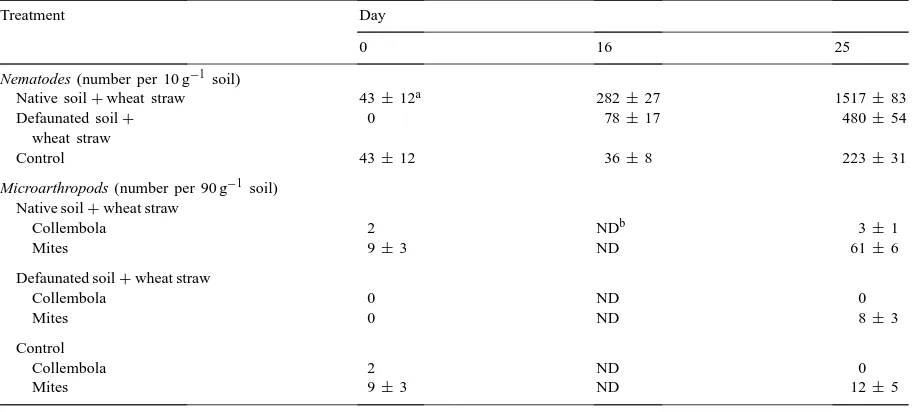
Abundance of soil micro and mesofauna during decomposition of wheat straw in a soddy-podzolic soil
Treatment Day
0 16 25
Nematodes (number per 10 g−1 soil)
Native soil+wheat straw 43±12a 282±27 1517±83
Defaunated soil+ wheat straw
0 78±17 480±54
Control 43±12 36±8 223±31
Microarthropods (number per 90 g−1 soil) Native soil+wheat straw
Collembola 2 NDb 3±1
Mites 9±3 ND 61±6
Defaunated soil+wheat straw
Collembola 0 ND 0
Mites 0 ND 8±3
Control
Collembola 2 ND 0
Mites 9±3 ND 12±5
aValue of standard deviation. bND: not determined.
3.4. Interrelation between bacteria, fungi and fauna
Soil fauna positively influenced the total microbial biomass, fungal biomass and CO2 evolution in soil
amended with starch and wheat straw after seven days of decomposition. The presence of soil animals may have increased the content of available nutrients (In-eson et al., 1982; Setälä and Huhta, 1991; McGo-nigle, 1995). Mechanisms of the stimulatory effects of the fauna on microbial growth and respiration may involve (1) the release of nutrients from the grazed, either consumed, non-digested, or digested microbial biomass (Coleman et al., 1983; Clarholm, 1985), and (2) the transport of limiting nutrients by animals to-wards decomposing plant residues. Migration of ne-matodes feeding on bacteria towards plant residues may accelerate the microbial biomass turnover (Grif-fiths and Caul, 1993). The grazing of soil animals may affect the physiological status of micro-organisms (Hedlung et al., 1991), which was indicated here by the metabolic quotient. Anderson (1987) considered that one third of total immobilised N might be re-leased as the result of faunal activity. The release of compounds from microbial biomass by grazing can explain accelerated decomposition rate of substrates when microbial growth depleted previously available nutrients. In addition, the fauna may kill rather than digest biomass, which is consistent with the observa-tion that microbial grazers have high consumpobserva-tion but low assimilation rates (Anderson et al., 1981).
Soil microbiological parameters were not appar-ently affected by predation during the first few days. However, microfauna significantly stimulated the res-piration rate of soil amended with starch and wheat straw after seven days (Fig. 2). The opposite effect of the microfauna occurred when microbial production was not restricted by available N, as indicated by the treatment with alfalfa meal. The CO2 evolution rates
and fungal growth were reduced approximately 25% by predation. Larsen and Jakobsen (1996) observed that the collembolan Folsomia candida reduced P transport by 75% in the mycelium of Glomus caledo-nium when soil was enriched with dry yeast. In the absence of soil Collembola, yeast addition increased AM-hyphal P transport by the development of an ex-tended hyphal net. They proposed that the interaction between F. candida and the external mycelium of G. caledonium was controlled by nutritional
condi-tions. It should be considered that invertebrates also consumed micro-organisms in non-amended soil but the predation under enriched conditions led to sup-pression of microbial growth. Schlatte et al. (1998) observed no significant effect of microarthropods on microbial production but did find increasing NH4+
content during decomposition of forest litter. The lack of stimulatory effects of soil fauna in their study may be due to ecological conditions favouring microbial growth, which became less controlled by grazing. Thus, nutrient status seems to be one of the most important factors determining the effect of soil fauna on microbial composition and activity.
The microbial biomass was also stimulated by the soil fauna when straw was added. Adding N-poor and N-free substrates to soil leads to microbial N immobil-isation. Cochran et al. (1988) demonstrated that micro-bial production was limited by N when leached wheat straw was added. Glucose amendment decreased the growth of pine seedlings due to microbial immobili-sation of available N and P (Bååth et al., 1978). Thus, available N may have initially restricted microbial growth during starch decomposition (Fig. 1). After seven days, predation on N-limited fungal and bac-terial biomass probably accelerated nutrient turnover and increased mineralisation rate. When soil was sup-plemented with alfalfa meal, microbial growth was less restricted by available N. Under those conditions, preferential consumption of active microbial cells as described by Klironomos and Kendrick (1995) led to the suppression of total soil respiration and fungal biomass. This is confirmed by the metabolic quotient (Fig. 2). The amount of CO2respired per unit of
component of total microbial biomass by the micro-faunal grazing agrees with the effect of soil macro-fauna (Anderson et al., 1985). Passage through the gut of the earthworm Lumbricus rubellus did not change significantly the total microbial biomass but did in-crease the bacterial component (Daniel and Anderson, 1992).
4. Conclusions
Addition of alfalfa meal, starch and wheat straw to a soddy-podzolic soil increased microbial and faunal growth in our microcosm experiment. The soil fauna reduced fungal growth when N-rich alfalfa litter was added to soil. Suppression of microbial production and mineralisation rate may have been due to exten-sive predation on active microbial biomass, which was not limited by nitrogen. Stimulatory effects of the soil microfauna on microbial biomass were most ob-vious during decomposition of N-poor substrates such as wheat straw and starch. We conclude that avail-able nutrients controlled microbial growth and the effect of the soil fauna can shift from suppression to stimulation.
Acknowledgements
This work was partially supported by the grant from the Russian Basic Research Foundation (Project no. 99-04-48000). We are grateful for the financial sup-port of the German Research Foundation (Project no. 436 RUS 17/73/99 and BL 91/35-1) and the state of Schleswig-Holstein that enabled the preparation of this manuscript. We want to thank all staff of the Ecology Centre in Kiel for their kind collaboration.
References
Alphei, J., Bonkowski, M., Scheu, S., 1996. Protozoa, nematoda and lumbricidae in the rhizosphere of Hordelymus europaeus (Poaceae) — faunal interactions, response of micro-organisms and effects on plant-growth. Oecologia 106, 111–126. Anderson, J.M., 1987. Interactions between invertebrates and
micro-organisms: noise or necessity for soil processes? In: Ecology of Microbial Communities, Cambridge University Press, Cambridge, pp. 125–145.
Anderson, J.M., Ineson, P., Huish, S.A., 1983. Nitrogen and cation mobilization by soil fauna feeding on leaf litter and soil organic matter from deciduous woodlands. Soil Biol. Biochem. 15, 463–467.
Anderson, J.M., Leonard, M.A., Ineson, P., Huish, S.A., 1985. Faunal biomass: a key component of a general model of nitrogen mineralization. Soil Biol. Biochem. 17, 735–737.
Anderson, J.P.E., Domsch, K.H., 1973. Quantification of bacterial and fungal contributions to soil respiration. Arch. Microbiol. 93, 113–127.
Anderson, J.P.E., Domsch, K.H., 1978. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 10, 215–221.
Anderson, R.V., Coleman, D.C., Cole, C.V., 1981. Effect of saprotrophic grazing on net mineralization. In: Clark, F.E., Rosswall, T. (Eds.), Terrestrial Nitrogen Cycles., Ecol. Bull., Vol. 33, pp. 201–216.
Bååth, E., Lohm, U., Lundgren, B., Rosswall, T., Soderstrom, B., Sohlenius, B., Wiren, A., 1978. The effect of nitrogen and carbon supply on the development of soil organism populations and pine seedlings: a microcosm experiment. Oikos 31, 153– 163.
Bardgett, R.D., McAlister, E., 1999. The measurement of soil fungal: bacterial ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol. Fertil. Soils 29, 282–290.
Brussaard, L., 1998. Soil fauna, guilds, functional groups and ecosystem processes. Appl. Soil Ecol. 9, 123–135.
Cairns, E.J., 1960. Methods in nematology. In: Sasser, J.N., Jenkins, W.R. (Eds.), Nematology, Fundamentals and Recent Advances with Emphasis on Plant Parasitic and Soil Forms, University of North Carolina Press, Chapel Hill, pp. 33–84. Clarholm, M., 1985. Interactions of bacteria, propozoa and plants
leading to mineralization of soil nitrogen. Soil Biol. Biochem. 17, 181–188.
Cochran, V.L., Horton, K.A., Cole, C.V., 1988. An estimation of microbial death rate and limitations of N or C during wheat straw decomposition. Soil Biol. Biochem. 20, 293–298. Coleman, D.C., Reid, C.P.P., Cole, C.V., 1983. Biological strategies
of nutrient cycling in soil systems. In: Macfadyen, A., Ford, E.D. (Eds.), Advances in Ecological Research 13, pp. 1–55. Daniel, O., Anderson, J.M., 1992. Microbial biomass and activity
in contrasting soil materials after passage through the gut of the earthworms Lumbricus rubellus Hoffmeister. Soil Biol. Biochem. 24, 465–470.
Dilly, O., Munch, J.C., 1998. Ratios between estimates of microbial biomass content and microbial activity in soils. Biol. Fertil. Soils 27, 374–379.
FAO, 1988. Soil map of the world. Revised legend. World soil resources report 60, FAO, Rome.
Griffiths, B.S., Caul, S., 1993. Migration of bacterial-feeding nematodes, but not protozoa, to decomposing grass residues. Biol. Fertil. Soils 15, 201–207.
Hanlon, R.D., Anderson, J.M., 1979. The effects of collembola grazing on micriobial activity in decomposing leaf litter. Oecologia 38, 93–99.
Hedlung, K., Boddy, L., Preston, C.M., 1991. Mycelial responses of the soil fungus, Mortierella isabellina, to grazing by Onychiurus
armatus (Collembola). Soil Biol. Biochem. 23, 361–366.
Heneghan, L., Coleman, D.C., Crossley, D.A., Xiaoming, Z., 1999. Nitrogen dynamics in decomposing chestnut oak (Quercus
prinus L.) in mesic temperate and tropical forest. Appl. Soil
Ecol. 13, 169–175.
Huhta, V., Setälä, H., 1990. Laboratory design to simulate complexity of forest floor for studying the role of fauna in the soil processes. Biol. Fertil. Soils 10, 155–162.
Huhta, V., Wright, D.H., Coleman, D.C., 1989. Charcteristics of defaunated soil. 1. A comparison of three techniques applied to two forest soils. Pedobiologia 33, 417–426.
Ineson, P., Leonard, M.A., Anderson, J.M., 1982. Effect of collembolan grazing upon nitrogen and cation leaching from decomposing leaf litter. Soil Biol. Biochem. 14, 601–605. Ingham, R.E., Trofymow, J.A., Ingham, E.R., Coleman, D.C.,
1985. Interactions of bacteria fungi and their nematode grazers: effect on nutrient cycling and plant growth. Ecol. Monogr. 55, 119–140.
Kandeler, E., Kampichler, C., Joergensen, R.G., Mölter, K., 1999. Effects of mesofauna in a spruce forest on soil microbial communities and N cycling in field mesocosms. Soil Biol. Biochem. 31, 1783–1792.
Kaneko, N., Mclean, M.A., Parkinson, D., 1995. Grazing preference of Onychiurus subtenuis (Collembola) and Oppiella
nova (Oribatei) for fungal species inoculated on pine needles.
Pedobiologia 39, 538–546.
Klironomos, J.N., Kendrick, B., 1995. Relationships among microarthropods, fungi, and their environment. Plant and Soil 170, 183–197.
Klironomos, J.N., Kendrick, W.B., 1996. Palatability of microfungi to soil arthropods in relation to the functioning of arbuscular mycorrhizae. Biol. Fertil. Soils 21, 43–52.
Larsen, J., Jakobsen, I., 1996. Interactions between a Mycophagous
collembola, dry yeast and the external mycelium of an Arbuscular mycorrhizal fungus. Mycorrhiza 6, 259–264.
Leonard, M.A., Anderson, J.M., 1991. Grazing interaction between a collembolan and fungi in a leaf litter matrix. Pedobiologia 35, 239–246.
Mamilov, A.Sh., Byzov, B.A., Stepanov, A.L., Zvyagintsev, D.G., 2000. Measurement of fungal and bacterial biomass in soil amended with plant residues, Eurasian Soil Sci., in press. McGonigle, T.P., 1995. The significance of grazing on fungi in
nutrient cycling. Can. J. Bot. 73, 1370–1376.
Neely, C.L., Beare, M.H., Hargrove, W.L., Coleman, D.C., 1991. Relationships between fungal and bacterial substrate-induced respiration. Soil Biol. Biochem. 23, 947–954.
Setälä, H., Huhta, V., 1991. Soil fauna increase Betula pendula growth: laboratory experiments with coniferous forest floor. Ecology 72, 665–671.
Setälä, H., Martikainen, N., Tyynismaa, M., Huhta, V., 1990. Effects of soil fauna on leaching of nitrogen and phosphorus from experimental systems simulating coniferous forest floor. Biol. Fertil. Soils 10, 170–177.
Schlatte, G., Kampichler, C., Kandeler, E., 1998. Do soil microarthropodes influence microbial biomass and activity in spruce forest litter. Pedobiologia 42, 205–214.
Sparling, G.P., West, A.W., 1988. A direct extraction method to estimate soil microbial C: calibration in situ using microbial respiration and14C labelled cells. Soil Biol. Biochem. 20, 337– 343.
Vance, E.D., Brookes, P.C., Jenkinson, D.S., 1987. An extraction method for measuring soil microbial biomass. Soil Biol. Biochem. 19, 703–707.
Van der Drift, J., Jansen, E., 1977. Grazing of springtails on hyphal mats and its influence on fungal growth and respiration. In: soil organisms as components of ecosystems. Ecol. Bull. Stockholm 25, 203–209.
West, A.W., 1986. Improvement of the selective respiratory inhibition technique to measure eukaryote: prokaryote ratios in soil. J. Microbiol. Methods 5, 125–138.
West, A.W., Sparling, G.P., 1986. Modifications to the substrate induced respiration method to permit measurement of microbial biomass in soil of differing water contents. J. Microbiol. Methods 5, 177–189.
Wright, D.H., Huhta, V., Coleman, D.C., 1989. Characteristics of defaunated soil. II. Effects of reinoculation and the role of the mineral component. Pedobiologia 33, 427–435.