1/38
POLYMERS
[Adopsi dari: Zbigniew D Jastrzebski, “The Nature And Properties of Engineering Materials”, John Wiley & Sons, ISBN 0-471-63693-2, 1987, CHAPTER 10.]
A large group of engineering materials of steadily increasing importance in industrial applications is composed of natural and synthetic organic polymers. Natural polymers such as starch and cellulose are the basic constituents of all plants, while proteins form the basis for all animal life. Advances in our understanding of the relation between the molecular structure of polymers and their chemical and physical properties make it possible to design and produce various polymeric materials of required characteristics for specific engineering applications. Three main types of commercial products are considered: plastics. rubbers, and fibers.
FORMATION OF POLYMERS
High polymers are formed by polymerization reactions that occur by two main mechanisms: addition polymerization and condensation polymerization .The name polymer is formed by adding the prefix “poly” to the monomer generic name, for example, polyethylene. When the monomer has a substituted parent name or a multiword name, the parentheses are used after the prefix “poly.” Thus we can write poly(vinyl chloride), poly(propylene oxide), and poly(chlorotrifluoroethylene). The parentheses are purposely omitted in common usage.
10-1 ADDITION POLYMERIZATION
Addition polymerization is defined as the reaction that yields a product that is an exact multiple of the original monomeric molecule. Such a monomeric molecule usually contains one or more double bonds that, by intermolecular rearrangement, may make the molecule bifunctional. Addition polymerization reactions usually proceed by a chain mechanism involving either free radicals or ionic catalysis. The reaction involves three steps: initiation, chain propagation, and termination (Equation 10-lb, 10-1c, and 10-1d). Initiation involves the dissociation of an initiator or catalyst into two free radicals (R) and addition to a monomer molecule M to form an active radical, R−M*.
Propagation or growth of the polymer chain results from successive addition of other monomers to the active group.
Propagation
Termination may occur because of collision between the active ends of two growing chains resulting either in their combination (coupling) or chain transfer mechanism, or by addition of terminator such as a free radical or another molecular species present in the system.
Termination
2/38
In all these reactions only bifunctional monomeric molecules are formed by the intermolecular rearrangement of the double bond present in the original molecule. This can result only in the formation of long chains.
Another kind of addition polymerization is copolymerization. Copolymerization is the simultaneous polymerization of two or more chemically different monomers that react to form a polymer containing both monomers linked in one chain. For example. GRS rubber is the product of copolymerization of butadiene and styrene.
In this long-chain polymer double bonds are still present that, on activation, are able to form crosslinks between the chains in further polymerization reactions.
10-2 CONDENSATION POLYMERIZATION
Condensation polymerization can be defined as the reactions between functional molecules that lead to the formation of a polymer with elimination of some small molecules, usually water. Condensation polymerization reactions proceed by a stepwise intermolecular mechanism. The following reactions between dicarboxylic acid and dihydroxy alcohol, resulting in polyesters, illustrate this process.
The resultant molecule reacts again in the same way with the dihydroxy alcohol molecule; the process repeats itself until linear chains of indefinite length are formed.
R and R’ stand for organic groups such as CH2, (CH2)n, and others. The resulting
3/38
Another reaction of polycondensation, which results in the formation of long linear chains, is used in the manufacture of nylon.
The resultant molecule can subsequently react with other molecules or either adipic acid or hexamethylenediamine, yielding a linear polyamide, characterized by the linkage —NH⋅CO—.
These reactions also show that a compound having two functional groups at the end can only produce long linear molecules.
Still another type of polycondensation reaction can be illustrated by the formation of a linear polycarbonate polymer.
Here, the HCl molecule is evolved as the result of condensation and the carbonate linkage
is formed. Phenol is an aromatic compound consisting of six-membered carbon ring of the benzene type in which one hydrogen atom is replaced by the hydroxyl group.
4/38
10-3 CONFIGURATION OF POLYMER CHAIN
During addition polymerization of a monomer A, the resultant polymer can be either a straight chain
Furthermore, the monomer molecules can react in one or more of the following ways: head-to-head, head-to-tail, and tail-to-tail. This is illustrated by the formation of vinyl polymers, which are obtained from vinyl monomers with the general formula
where X may stand for any atom or group, such as H, Cl. F, CH3, etc. The mechanism of
polymerization involves adding a free radical R’ to the monomer in two possible ways:
Form I would lead to the formation of a head-to-tail arrangement in which the substituents occur on the alternate carbon atoms as shown by
A combination of forms I and II may lead to the tail-to-tail or head-to-head arrangement:
The possibility of obtaining only a regular head-to-head or tail-to-tail arrangement is relatively remote. Usually a mixture of all these arrangements occurs.
5/38
correspondingly, greater differences in polymer properties may result. Depending on the reactivities of monomers A and B on their relative proportions, different copolymers are formed. 1f the two monomer units alternate in random statistical distribution in the polymer chain random polymers result:
Alternating copolymers are formed when the A and B units are placed alternately along the polymer chain:
Another possibility is the formation of a graft copolymer, which is essentially a branched-chain structure having side chains composed of one type of the monomer unit attached to the backbone chain from another monomer unit:
Such a graft copolymer can be produced either by prepolymerizing monomer B and grafting it onto the main backbone chain consisting of the monomer A.
or by polymerizing in situ where a molecule first attaches to the backbone chain A and forms a “grafted-on” section:
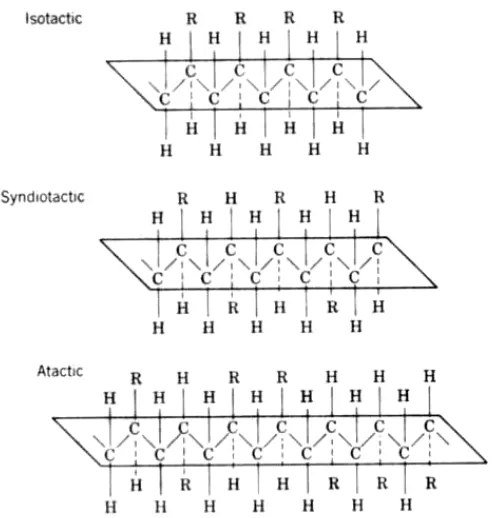
Stereoregular Polymers . Using special stereospecifïc catalysts, like Ziegler-Natta, it is
possible to control the stereoregularity of the polymer chain by varying the type of initiator and the polymerization conditions. For example, a polymer chain of the composition contains asymmetric carbon atoms (C*) holding bulky R groups.
The R group may stand for CH3, C2H5, C6H5, and the like. If the R groups are all located
6/38
polymer is obtained (Fig. 10-1). Finally, if the groups are oriented at random then the polymer has an atactic arrangement and it is amorphous. Both isotactic and syndiotactic arrangements produce crystalline polymers (Fig. 10-1).
FIGURE 10-1 Stereoisomers of a polymer chain having a bulky group R along the backbone chain.
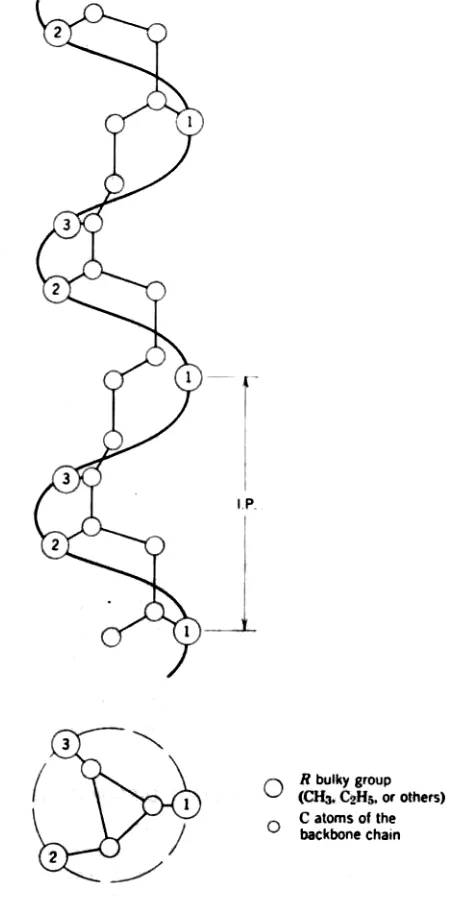
The bonds between the repeat units of a polymer chain are relatively flexible to permit rotation of the groups about the bond. This may result in various helical conformations of the chain to attain their close packing. The distinct rotational states of the groups that stabilize the helical conformation are the trans position and the two gauche positions. The trans and two gauche forms are alternately located along the backbone polymer chain to relieve any steric hindrance that might arise because of the bulky group. For two gauche positions left or right, either a left-hand helix or right-hand helix is formed (Fig. 10-2). A polymer helix is usually characterized by the identity period, I.P., and by the number of monomeric or repeat units in this period. For example, the propylene helix has an identity period of 650 pm and the number of repeat units per turn is 3. If the side groups are very bulky more space is required and the resultant helix may contain more than three repeat units per turn, forming much looser helices.
10-4 MOLECULAR WEIGHT DISTRIBUTION
7/38
FIGURE 10.2 Helical conformation of an isotactic vinyl polymer. IP is the identity period of the repeat distance of the helix containing three
repeat units. Hydrogen atoms are not shown.
As the molecular weight of the polymer is related to its chain length or to the degree of polymerization, it can be defined as
where Mm is the molecular weight of the monomer and DP denotes the degree of
8/38
Number-average molecular weight, Mn, is the weight of a polymer sample divided
directly by the number of molecules in this sample. Mathematically this is expressed as
Number-average molecular weights are obtained from such measurements as osmotic pressure, boiling point elevation, and freezing point depression. In all these methods the number of molecules for each fraction is counted in a known mass of the polymer and, through Avogadro’s number, the number-average molecular weight is estimated.
Weight-average molecular weight, Mw , is defined as the weight fraction of wi of the
polymer chains times their corresponding molecular weight Mi divided by the total
weight of the polymer sample investigated. This can be represented by the relation
where wi is the weight of fraction i having mean molecular weight Mi, and ni is defined
as before. The weight-average molecular weight is usually determined by light scattering, which depends on the size and the mass of the molecule.
Number-average and weight-average molecular weights are most frequently used in characterization of the molecular weight and the molecular weight distribution of the polymer. Another two averages occasionally used are z-average molecular weight, obtained by sedimentation in an ultracentrifuge and defined as
and viscosity-average molecular weight, defined by
where K and a are constants to be determined experimentally. The term [η] is specific
viscosity of a polymer solution, which is determined from
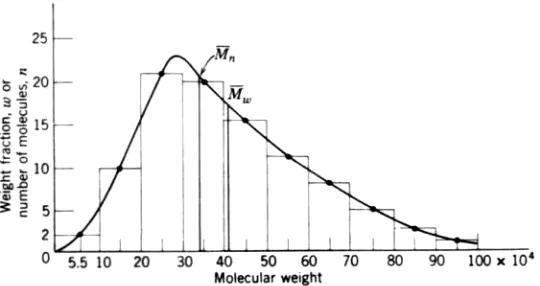
The curve representing the number and weight fraction of the molecular-weight distribution is given in Fig. 10-3, which shows the number- and weight average molecular weights. For a polymer with all chains of the same length all four averages will be the same:
Frequently, the ratio of the weight-average molecular weight to the number average molecular weight, Mw/Mn is used to determine the spread of the molecular weight
distribution of the polymer. For a narrow molecular weight distribution the ratio
n
w M
M / is close to one but, for a broad molecular weight distribution, it may be as high
9/38
Illustrative Problem 10.1
10/38
11/38
LINEAR POLYMERS
Linear polymers form the largest group of plastics covering a great variety of diversified products used in different forms and applications. Linear polymers can be obtained by either addition polymerization or condensation polymerization of bifunctional monomers. Being thermoplastic, they can be easily worked into required forms and shapes at elevated temperatures.
The structure and properties of linear polymers depend on the chemical nature of the monomer, the geometry of the polymer chain, and the magnitude of the intermolecular forces between the chains. These intermolecular forces depend on the molecular weight or the chain length, the presence of polar groups and their spacing and regular distribution along the backbone chain, the possibility of formation of the hydrogen bond, and the distance between chains. The structural regularity of the chain determines the degree of packing of the chains and its state, either amorphous, crystalline, or semicrystalline.
10-5 DEGREE OF CRYSTALLINITY
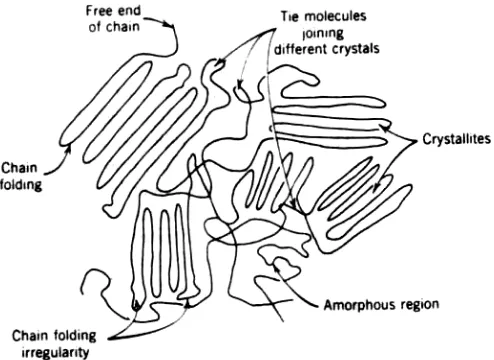
Polymers crystallized from melt on cooling of different degrees of perfection consist of individual single crystal lamellae connected to each other through tie molecules, which may meander randomly through the disordered regions before participating in the formation of another chain-folded crystal (Fig. 10-4). We can therefore consider such a crystalline polymer as a two-phase system consisting of an amorphous phase and an ordered crystalline phase that differ in their density and other physical characteristics. Crystallization causes a denser packing of molecules, increasing the intermolecular forces. The degree of crystallinity may range from 0% for noncrystallizable polymers through intermediate crystallinities such as 20% for poly(vinyl chloride), 50% for branched polyethylene, and up to 95% for polytetrafluoroethylene (TFE) and a linear polyethylene.
FIGURE 10.4 Schematic representation of the typical crystalline polymer showing randomly arranged crystalline and amorphous regions. A polymer chain
may go through several crystalline and amorphous regions.
12/38
normal amorphous polystyrene of similar molecular weight has a softening point of only 90°C (190°F). Similarly, branched-chain polyethylene, which is only up to 60% crystalline, has a density of 910 to 930 kg/m3 (0.910—0.930 g/cm) and a melting point
of 115°C (240°F). whereas linear polyethylene, considered to be 90 to 95% crystalline, has a density of 940 to 965 kg/rn3 and a melting point of 135°C (275°F).
The density of perfectly crystalline material can be obtained from X-ray measurement, while that of the amorphous material can be easily determined above its melting point and extrapolated to room temperature. Thus the percentage crystallinity can be calculated from the relation
The degree of crystallinity and the amount of amorphous region can be controlled by copolymerization, which lowers the structural symmetry of the polymer chain, thereby decreasing the crystallization tendency. For example, vinylidene chloride is usually copolymenzed with about 10% to 15% vinyl chloride to produce a material of greater flexibility than the pure vinylidene polymer. A copolymerization is a normal procedure in producing rubberlike polymers. A crystalline or amorphous polymer can be made more flexible by adding plasticizers.
Crystallinity of a polymer is also controlled by its cooling rate. At fast cooling rates, even a strongly crystalline polymer will not be able to crystallize and will form an amorphous structure. Many polymers that crystallize more slowly can be quenched to a glassy amorphous solid and, if their Tg is sufficiently high, as in the case of a polyamide
(nylon 66) or isotactic polystyrene and poly(ethylene terephthalate), they may remain amorphous at room temperature for an indefinite period of time. On the other hand the rate of crystallization in polyethylene and Teflon is so high that crystallization cannot be prevented by quenching the melt, even in liquid nitrogen.
10-6 EFFECT OF POLAR GROUPS
The presence of a polar group
in the monomer increases the intermolecular forces between the chains, resulting in a higher softening point and a greater stiffness and rigidity of polymer, as exemplified by poly(vinyl chloride) compared with polyethylene. A normal poly(vinyl chloride), although much less crystalline than polyethylene because of the introduction of a chlorine atom, which lowers the symmetry of the chain, is stronger than polyethylene:
13/38
structures produce polymers of high melting points, high rigidity, and tenacity. The polyamides and polyterephthalates have a molecular weight only around 20,000. This value is an order of magnitude lower than that of other plastics, such as polystyrene. poly(methyl methacrylate), poly(vinylchloride), and polyethylene, which would have very low strength with such a low molecular weight. This shows clearly that the excellent strength of polyamides is due to the strong secondary valences caused by the presence of the regularly ordered polar groups. Thus, in nylon 66, the frequency of the polar groups —CO⋅NH-—- and the formation of 0 ... H—N hydrogen bonds between the chains leads to maximum interaction between the chains, which accounts for a high degree of crystallization and a strong, rigid material with a melting point of 260°C (500°F). Another example of the effect of the numerous polar groups and hydrogen bonding between the chains is given by natural polymer of cellulose. Cellulose, a main substance of all plants, is composed of anhydroglucose groups linked together to form a long chain having from 3000 to 4000 glucose unit:
The structure shows three hydroxyl groups on each glucose unit. The hydrogen-bonded structure of cellulose and its molecular weight account for the very strong intermolecular forces between the chains. These give high rigidity and crystallinity to the polymer, preventing it from melting and dissolving below its decomposition temperature. To make the cellulose more amenable for manufacturing operations, it is necessary to decrease these intermolecular forces by reducing the molecular weight or by neutralizing the polar character of the hydroxyl groups.
Regenerated cellulose is essentially the same chemical compound as natural cellulose, but it has a much lower molecular weight, containing only from 300 to 500 glucose units. This lower molecular weight decreases greatly the intermolecular forces between the individual chains, making it possible to obtain cellulose in a solution form. This reduction of the molecular weight is accomplished by special chemical treatments with sodium hydroxide and subsequent treatment with carbon disulfide to produce a heavy viscous solution, known as viscose. The viscose can be spun to give fibers or extruded through a die for film. Then the cellulose is regenerated from this viscous solution by precipitating it in an acid batch as fibers (viscose rayon) or as film (cellophane), If the hydroxyl groups are replaced by acetate groups in the natural cellulose as the result of an esterification reaction with acetic anhydride and glacial acetic acid so that about 2 to 2.5 acetyl groups per glucose residue remain, cellulose acetate is produced. Cellulose acetate is no longer soluble in sodium hydroxide, but it is soluble in acetone. Complete substitution of three acetyl groups per glucose unit, however, makes the polymer insoluble in acetone. Cellulose acetate can be spun from viscous solution in acetone as flbers or used with plasticizers to form various plastic products and films.
14/38
The requirements of high tensile strength and high melting point (usually above 200°C (400°F)) require a high cohesion energy associated with a high degree of crystallinity. Thus the characteristic feature of a linear polymer to be a good fiber-making material is a high geometrical symmetry of the polymer chain and high intermolecular forces between the chains. Branching is not desirable because it disrupts the crystalline lattice and lowers the crystallinity.
The crystallization of a polymer can be enhanced by its exposure to a shear gradient, as in stretching or drawing the solution or the melt of the polymer during or after cooling. This is used in the production of synthetic fibers and films. The melt or solution is first squeezed through a thin spinneret or a die, and the resulting fiber or film is stretched while being cooled or the solvent from viscous solution is evaporated.
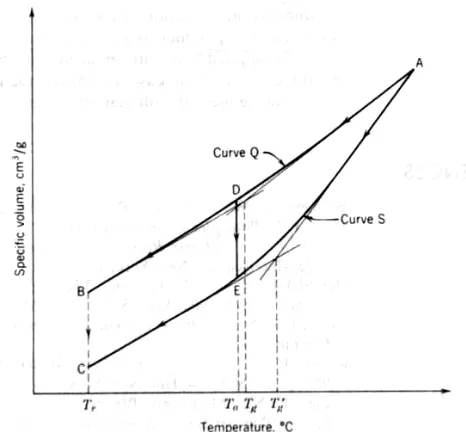
10-7 TRANSITION TEMPERATURES
Since crystalline polymers are never completely crystalline and contain both ordered (crystalline) regions and unordered amorphous regions, depending on the structure and thermal history of the polymer, they usually exhibit two characteristic temperatures: the melting point, Tm, defined as the temperature at which crystalline
aggregates disappear, and the glass transition temperature, Tg.
The glassy state of polymers is a rigid and brittle noncrystalline state. However, the brittle characteristics vary widely from polymer to polymer and with temperature. The glass transition temperature arises because of hindered relaxation of the chain molecules as the volume decreases with the temperature. Although the translational movement of the chain segment can be frozen and the rotation of the bulky groups may be stopped, some degree of vibration and local motion of the atoms may occur. Thus many polymers have other secondary or tertiary transitions in addition to the main glass transition. For example, crystalline polytetrafluoroethylene (TFE) shows four different transitions below its melting point of 327°C the main glass transition at + 127°C and the other three glass transitions at +30 −19, and −97°C. Polycarbonate has a glass transition of 150°C but it is a strong, rigid polymer having exceptional impact resistance at temperatures much below Tg. This appears to be due to the local
motion within the carbonate linkage
which persists up to −110°C and is capable of absorbing energy upon impact loading. Another example can be provided by nylon 66, which has a main glass transition at about 50°C, but retains a crankshaft motion of its methyl groups within the chain up to a temperature of −120°C.
Polymers may also exhibit the decomposition temperature, Td, at which the
15/38
temperatures (Tm, Tg, and Td) define the range of applicability of the polymers in
practice. Rubbers are used between Tg and Td, whereas amorphous polymers are used
below their glass transition temperature, but they must be formed and shaped between Tg and Td, where they are already soft and rubbery. Crystalline polymers can
be used up to their Tm; they are hot formed and shaped between Tm and Td and cold
formed between Tg and Tm. Because of the complex structure of crystalline polymers
and the many defects in the crystalline regions, their melting temperature is usually within a few degrees of the thermodynamic melting temperature, Tm ====∆Hf /∆Sf .
The heat capacity, coefficient of expansion, diffusion coefficient, and elastic moduli undergo rapid changes in going through the glass transition. The mechanical damping of low-frequency oscillations exhibits a sharp peak in the region of Tg. The
glass transition temperature increases with the increase in intermolecular forces, molecular weight, crosslinking, and bulky and side group substituents that restrict rotation. For crystalline polymers the glass transition temperature is related to the melting point:
For symmetric polymers Tg/Tm ≈ 0.5, whereas for unsymmetrical polymers such as
poly(trifluorochloroethylefle) or propylene, the ratio Tg/Tm is about 0.67. The glass
transition temperature is lowered with the addition of plasticizers.
Below Tg, the segments of an amorphous polymer undergo vibratory motions around
fixed positions, while above Tg, the segments exhibit translational and diffusional
motions. Above Tg. linear amorphous polymers exhibit time-dependent behavior. At
a small constant stress, they show an elastic response, a retarded recoverable response, and finally, a non-Newtonian flow. Amorphous linear (thermoplastic) polymers show the glass transition temperature which may vary from 40 to 150°C (104—300°F) and sometimes higher, depending on the type and the structure of the polymer.
For high molecular weight polymers, the solid is glassy below Tg but, as the
temperature gradually increases, it becomes leathery, rubbery and, finally, liquid. For low molecular weight polymer, the polymer changes from glassy below Tg to liquid
above Tg. The major factor determining the value of Tg is the flexibility of the
polymer chain. Steric hindrance and bulkiness of the side groups attached to the backbone chain usually cause an increase in Tg.
The viscoelastic behavior of a polymer as a function of temperature is illustrated in Fig. 10-5, which shows the changes in modulus elasticity versus temperature for different structural arrangements.
There are five regions of viscoelastic behavior of the amorphous polymer. The glassy state below Tg characterized by a steady value of the modulus of elasticity, the
glass transition region Tg where the modulus drops rapidly, the rubbery plateau
16/38
FlGUR 10-5 Changes of the modulus elasticity with temperature at constant time of 10 s for different structural arrangements. Er(10) is the relaxation tensile modulus
during 10 s at a particular temperature. Curve A represents an amorphous polymer of low molecular weight, B is an amorphous polymer of high molecular weight, C is the crystalline polymer, and D is a crosslinked polymer. (After A. V. Tobolsky, Propemes and Structure of Polymers, John Wiley & Sons, Inc.. New York, 1960.)
CROSSLINKING IN POLYMERS
Crosslinking usually involves the introduction of a covalent type of link between polymer chains or their segments. An initially small amount of cross-linking causes formation of some branched molecules that still are soluble but, on further reaction, gelation sets in. This stage is characterized by the presence of insoluble gel and the soluble sol, which can be extracted from the gel. On further crosslinking a giant three-dimensional network is formed that imparts rigidity, infusibility, insolubility, and improved heat resistance to the polymer. If the crosslinks are short and densely located, hard and strong polymers are obtained that exhibit little elongation and high moduli. Crosslinking may occur through (1) functional groups, (2) addition at the double bond of the polymer or to the reactive groups located along the polymer chain, (3) radical formation, and (4) secondary valences, such as vander Waals forces, dipole-dipole interaction, hydrogen bond, and ionic bonding.
10-8 CROSSLINKING THROUGH FUNCTIONAL GROUPS
This mechanism of crosslinking involves a condensation reaction that follows exactly the same path as that of the original polycondensation reactions during the formation of the polymer macromolecule. For this reason the term thermoset is applied to such crosslinked polymers, since their final curing after application is carried out under a pressure adequate to prevent the evolution of water molecules formed during crosslinking from the system.
Phenol-Formaldehyde (Phenolic) Resins. Phenol-formaldehyde resins, simply
17/38
catalyst, the temperature, and the time of reaction. Two quite separate phenolic resins are produced:
With two or three molecules of formaldehyde we obtain
The reaction between methylol phenols and phenol may occur either (1) between the methylol hydroxyl group and the hydrogen in the benzene ring, or (2) between the two methylol hydroxyl groups. In both cases the water molecule is split off:
Further heating produces a linear polymer of varying length:
A bond through the radical —CH2— in Reaction 10-37 is called the methylene linkage; the bond through the radical —CH2—O—CH2— in Reaction 10-38 is called the ether linkage. In the one-stage process, phenol is reacted in the presence of alkaline catalysts with an excess of formaldehyde, so that the phenol-to-formaldehyde ratio (P/F) is less than one. The reaction is stopped when the product is still soluble and fusible, producing either A-stage or B-stage resin. The A-stage resin, called resol, is a relatively short, low molecular weight, linear polymer, which is completely soluble in the alkaline solution present in the reaction vessel. The B stage, called resitol, is a rather long, linear polymer with a slight amount of crosslinking between chains; it is insoluble in alkaline so lutions but readily soluble inorganic solvents and it is fusible. Resin A or B, or the combination of both, is used for adhesives, casting, plastics, and laminates.
18/38
with proper ingredients such as fillers, stabilizers. lubricants, and dies. A crosslinking agent (hexamethylenetetramine) is added to provide the source of —CH2— links for subsequent crosslinking reactions. Final curing of either A- or
B-stage resin or Novolac, which is carried out after the application of material, gives a three-dimensional network of densely spaced but relatively short crosslinks, designated as C-stage resin:
Phenolic resins find numerous and varied industrial applications as adhesives, casting, coatings, laminates, and structural products with various fillers and fibers where high rigidity, corrosion resistance, and heat resistance are required.
Amino ResIns. Similar mechanisms of reactions occur during polycondensation of
urea-formaldehyde and melamine-formaldehyde, producing various amino resins known as urea and melamine resins:
10-9 CROSSLINKING THROUGH ADDITION
Crosslinking through addition may involve reaction of an unsaturated compound between two polymer chains containing a polymerizable double bond, as in unsaturated polyesters or some rubbers. Another addition reaction may occur between a compound (catalyst) and the active end group of prepolymer chains, as exemplified by curing epoxy and polyurethane resins. In the first case the covalent crosslink is formed through the rearrangement of double bonds in the crosslinking agent as well as in the polymer. In the second case the crosslink results from the rearrangement of atoms in the end groups so that a simple addition of the catalyst molecule to both chains becomes possible. In both cases no molecule is evolved as the result of crosslinking reactions. This makes possible easy casting of the prepolymer resin with the catalyst (crosslinking agents) and various additives such as reinforcing agents under normal atmospheric pressure.
Unsaturated Polyesters. Linear, or saturated, polyesters as mentioned before, are typical
19/38
A vinyl-type compound of general formula
is used for crosslinking of the linear chains, as shown below. The reaction occurs by simple addition. The extent of crosslinking can be controlled by varying the amount of unsaturation in the polyester and the amount and kind of crosslinking agent. For example, an unsaturated polyester crosslinked with styrene is harder and tougher than that crosslinked with methyl methacrylate. Unsaturated polyester resins are widely used as glass-fiber reinforced materials for various structural applications.
Epoxy Resins. Epoxy resins are the combination of bisphenol A and epichlorohydrin.
Which leads to the formation of a relatively short chain linear polymer containing two reactive groups, epoxide and hydroxyl:
20/38
It is also possible to carry out a wide range of crosslinking reactions with other polymeric resins such as amines, phenolics, polyamides, cellulose, and vegetable oil fatty acids, producing a great variety of products of specifically modified properties. Because the ether linkage is a very stable one, epoxy resins have high chemical resistance to water, various solvents, acids and alkalies, and other chemicals. The reactive groups are comparatively widely spaced, resulting in high flexibility, but, at the same time, the presence of crosslinks accounts for the toughness and heat resistance of the cured polymer. Furthermore, the polar nature of such groups as epoxide and hydroxyl contributes to good adhesion. Epoxy resins are most frequently used as coatings, adhesives, and glass-fiber reinforced plastics.
10-10 CROSSLINKING BY FREE RADICALS
This crosslinking may be accomplished by using free radicals that being a very active species, can attach themselves between the polymer chains, forming covalent links. Such free radicals can be formed as the result of intensive irradiation or by chemical action on the prepolymer, creating active centers along the backbone chains, or by using highly reactive compounds as organic peroxides and azo compounds. On heating, the latter decompose, giving free radicals.
Crosslinking by Irradiation.
Radiation-induced crosslinking occurs as the result ofthe impact of high-energy radiation on the polymer chains, which causes knocking out of hydrogen atoms and produces secondary free radicals on the polymer chain. This makes possible the formation of covalent links between the polymer chains. Crosslinking is generally found to occur more readily in the amorphous regions between polar crystallites.
High-energy irradiation results in either crosslinking or chain scission, depending on the chemical structure of the polymer, and on the dose of radiation. Polymers that crosslink are polymethylene, polyethylene, polypropylene, polystyrene, polyvinyl chloride, polyamides, polyethylene siloxane, and others. Polymers that disintegrate are polybutylene, polytetratluorethylene, poly(methylmethacrylate), cellulose derivatives, and others. High-energy radiation is also used to initiate polymerization in grafting polymer on another polymer as polystyrene and rubber.
Crosslinking by Organic Peroxide. Organic peroxides are frequently used in the curing
21/38
Free radicals abstract hydrogen atoms from methyl groups of the two polymer chains forming a crosslink between them.
where R* stands for C6H5COO* or C6H5*.
10-11 CROSSLINKING THROUGH SECONDARY VALENCES
We can extend the concept of crosslinking through secondary valence forces, especially groups such as —COOH and —CONH2 which, because of hydrogen bond formation,
are especially tight together. Such secondary valence cross-linking does not always have to be caused by hydrogen bonds. In principle all groups with high molar cohesion are capable of secondary valence cross-linking, especially polar groups such as —COOH and —SO3H.
Secondary valence crosslinking differs from covalent crosslinking in that it disappears on heating. This makes the polymer thermoplastic in nature and accounts for its easy processability as compared to completely crosslinked covalent materials. This secondary crosslinking can also be removed by treatment with suitable polar solvents such as dimethylformamide. After removal of the solvent during wet spinning, the resulting threads behave as vulcanized rubber. Secondary valence crosslinking plays a highly important and decisive role in nature; all proteins are reversibly crosslinked through the —CONH— groups. A similar type of crosslinking can be found in polyelectrolytes, also called ionomers. lonomers are copolymers derived from ethylene and methacrylic acid in which the ionized carboxylic groups form ionic crosslinks in the intermolecular
structure:
The ionized groups are neutralized by zinc or sodium ion.
10-12 ELASTOMERS
Elastomers are an important group of polymeric materials that are subject to many crosslinking processes to impart desired properties to the rubber. Any linear polymer can be made a good rubber if it meets the following characteristics.
1. The polymer chain should be very long and geometrically irregular so that thermal agitation will result in a strongly entangled and coiled-type arrangement.
2. The intermolecular forces between the polymer chains should be such that at room temperatures thermal energy is sufficient to maintain them in a state of constant mobility. This is comparable to the statement that the glass transition temperature should be below the working range of the temperature for a rubber.
3. There must be a possibility for introducing crosslinks between the chains so that the required degree of rigidity can be obtained.
22/38
regularity of the chain and give a long chain of relatively weak intermolecular attraction. The presence of polar groups is usually avoided unless special characteristics such as oil resistance and improved heat resistance are required at the expense of flexibility of the polymer chain.
Vulcanization.
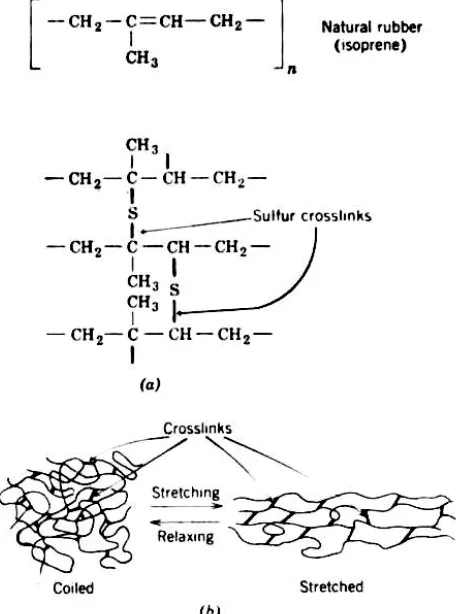
Vulcanization is the term used in the rubber industry to refer to thevariety of crosslinking processes used. Natural and synthetic rubbers such as styrene— butadiene (SBR), polybutadiene, acrylonitrile, and others contain a double bond capable of crosslinking (see Appendix A7). The mechanism of such crosslinking is essentially identical with crosslinking through addition polymerization, as illustrated by the curing of unsaturated polyesters. However, in a typical rubber, there is only about one crosslink to every few hundred chain atoms.
FIGURE 10-6 Vulcanization of natural rubber. (a) Mechanism of crosslinking of isoprene molecules by means of sulfur atoms between two carbon atoms with double
bonds (b) Schematic representation of transition from the randomly crosslinked coiled snarls to the oriented state on stretching. The presence of crosslinks causes the
chain molecules to return to their previously coiled conformation on relaxation of stress, thereby preventing the permanent set.
23/38
chains become oriented (Fig. 10-6b). This orientation results in crystallization that increases the attraction forces between the chains, causing the material to stiffen. When the force is released, strained bonds are allowed to return to the original random snarl arrangement of the molecules. Such changes in the molecular configuration account for characteristic elongation of rubbers on stretching and their contraction (unloading) on the release of force (see also Fig. 7-9). Some rubbers, especially the natural rubber, crystallize easily on stretching. considerably improving their tensile strength. On the other hand, synthetic rubbers such as styrene—butadiene rubbers do not crystallize readily when stretched. They show poor tensile strength unless reinforced with fillers such as carbon black.
Speciality Rubbers. The presence of the double bond in the polymer chain results in the
rather poor oxidation resistance of the rubber. To improve that, new synthetic rub bers such as chiorosulfonated polyethylene, silicones, polyurethanes, and fluororubbers have been developed that have a completely saturated backbone chain. For crosslinking, special reactive groups are provided at certain intervals along the main chain, or specially active compounds such as peroxides, producing free radicals on heating, are used.
For example, chlorosulfonated polyethylene, known as Hypalon, is produced by reacting polyethylene with chlorine and sulfur dioxide. This results in the introduction of chlorine atoms and a small number of chlorosulfonyl groups. —SO2Cl, into the polyethylene
chain. A random spacing of chlorine atoms in the chain reduces the crystallinity of the polyethylene, and chemically active chlorosulfonyl groups form crosslinks with vulcanizing agents such as PbO or MgO. The reaction proceeds as follows:
R stands for the chain repeat unit of Hypalon.
and crosslinking can be schematically shown by
Since the rubber has no unsaturated bonds, it shows high oxidation resistance and high chemical resistance.
24/38
irregular chain possessing active isocyanate groups for crosslinking. This can be accomplished using glycols, producing the isocyanate linkage:
Water can also be used producing the urea linkage in two steps with the elimination of CO2:
Reactions 10-55a and 10-55b are used to produce various polyurethane foams with water. The final structure of the polyurethane can be represented schematically by
where—B—designates the prepolymer, which can be a polyester, or a polyether, or a polyester—polyamide. Polyurethanes are highly resistant to oxidation because of their saturated character; they also show good resistance to many organic solvents, but they are attacked by acids and alkalies, especially hot or concentrated, similarly to polyamides. Isocyanates are also used to produce fibers as well as other resins, which are characterized by strong adhesion to many material surfaces.
10-13 THERMOPLASTIC ELASTOMERS
Thermoplastic elastomers are the block copolymers which exhibit the elastic properties of elastomers at the use temperature but can be processed as thermoplastics at elevated temperatures. Usually. thermoplastic elastomers are two-phase block copolymers containing both hard and soft polymer segments. The hard segments form domains which, at service temperature. prevent the flow of the soft elastomeric segments but, at elevated temperatures. soften and plasticize the rubber segments so that the material can be processed as a conventional thermoplastic. There are two main types of thermoplastic elastomers: modified thermoplastic elastomers and thermoplastic elastomers by polymer blending.
Modified Thermoplastic Elastomers. The modified thermoplastic elastomers are blends
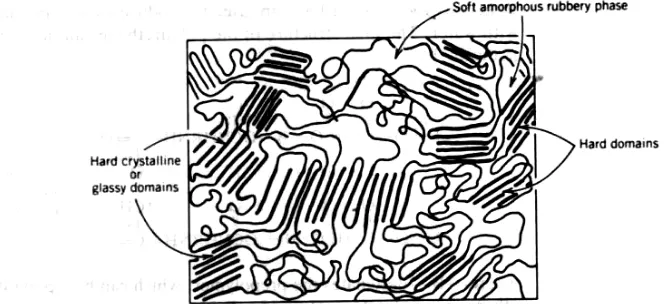
of homogeneous polymers having various types of temporary crosslinkings such as glassy block polymers of polystyrene (PS) in styrene—butadiene—styrene (SBS) copolymers or the crystalline hard phase of tetramethylene terephthalate in a thermoplastic copolyester/polyether (Fig. 10-7).
25/38
FIGURE 10-7 Schematic diagram of a thermoplastic elastomer morphology. Hard crystalline or glassy domains function as crosslinks for soft amorphous rubbery phase.
Thermoplastic polyester elastomers (TPE) and thermoplastic polyurethane elastomers (TPU) are alternating block copolymers having segments of a hard. highly polar or crystallizable material linked by segments of amorphous (soft) rubber-like material at normal service temperature. in TPE the crystalline block consists of dimethyl terephthalate segments and the amorphous soft rubbery phase is poly(tetramethylene ether) glycol terephthalate segments randomly distributed along the polymer chain. Crystalline regions are about 10 nm (100 Å) wide and several hundreds nanometers long. The melting point of these domains is around 200°C (400°F).
In TPU the hard, highly polar blocks of 4.4-phenylmethane diisocyanate or 2,4—2,6-toluene diisocyante extended with 1,4-butane diol form hydrogen-bonded crystalline or glassy domains and the soft elastomeric phase consisting of polyol segments.
TPU and TPE are polar materials and the choice of polymers for blending is limited to polar polymers such as PVC, ABS. and polyesters. Such blending improves abrasion resistance and flexibility and gives a product that is less expensive than pure thermoplastic elastomers. Because of their crystalline blocks both TPU and TPE materials show good resistance to fuels, oils, and similar products. TPU are characterized by exceptionally high tensile strength up to 50 MPa (7 ksi), large hysteresis, and stress softening.
Thermoplastic Elostomers by Polymer Blending. The most useful thermoplastic
elastomers under this group are polyolefin blends of a crystalline polyolefin such as isotactic propylene or less frequently polyethylene (HDPE) with a polyolefin rubber such as an ethylene—propylene copolymer (EDR) or ethylene—propylenediene terpolymer (EPDM). The rubbery phase is slightly crosslinked during mixing to enhance elastic recovery. At higher temperatures, the crystalline phase melts and flow of the mixture becomes possible. Upon cooling, the crystalline phase rehardens and the original properties return. Other examples of this class of thermoplastic elastomers are butyl rubber—polyethylene blends and silicone—polyethylene blends.
10-14 INTERPENETRATING POLYMER NETWORK
26/38
initiation in the presence of a polyurethane network. The possibility of combining various chemical types of polymeric networks has produced IPN compositions that exhibit synergistic behavior. If one polymer is elastomeric in nature and another is glassy, then a reinforced rubber is obtained if the elastomer phase predominates, and an impact-resistant plastic results if the glassy phase predominates.
There are a few categories of interpenetrating networks. When only one polymer is crosslinked and the other is linear the product is called a semi IPN. Semi-1-IPN or semi-2-IPN exists when, respectively, polymer 1 or polymer 2 is the crosslinked component. Furthermore, in addition to IPN—the general term for interpenetrating polymer network—we can distinguish the simultaneous interpenetrating network (SIN), where both polymers are synthesized simultaneously, by either addition or condensation polymerization reactions, and the interpenetrating elastomeric network (IEN). The latter refers to those materials that are made by mixing and coagulating two different polymer latexes and crosslinking the coagulum to form a three-dimensional structure. If the latex coagulum is not crosslinked, the resulting product is called a latex polyblend.
GENERAL PROPERTIES
10-15 HIGH-TEMPERATURE RESISTANCE AND THERMAL STABILITY
A serious drawback of polymers as engineering materials is their relatively poor heat resistance and low thermal stability. A considerable research effort has been made in developing new polymers of improved thermal stability and heat resistance. The progress has been achieved along two main directions: developing aromatic polymers and developing inorganic polymeric materials.
Aromatic Polymers. Polymers having more cyclic rings in their chains are stiffer and
more resistant to deformation because segmental rotation of the chain segments becomes more difficult. Hence the melting points and the glass transition temperatures increase, and the solubility and the deformation-under-load at elevated temperatures decrease. The presence of aromatic rings improves the thermal stability of a polymer still more, since aromatic rings are thermally very stable by nature. The application of these principles in creating new polymers has brought remarkable improvement in heat resistance and oxidation resistance of some specific polymeric materials. Examples of this are polybenzimidazole and polyimides, which show heat resistance for a short time up to a temperature of about 600°C (1112°F).
The other way to provide good heat resistance is to use the inorganic material, as exemplified by silicone polymers. as a backbone chain. Silicone polymers are based on an inorganic silicon—oxygen structure that has organic radicals attached to silicon atoms. Because of the presence of silicon—oxygen links, they exhibit outstanding heat resistance, but their chemical resistance is generally lower than that of other plastics. Two or more functional groups can be attached to the tetravalent silicon atom that, on polycondensation, may lead to chain or crosslinked polymers (Table 10-1). The low molecular weight silicone polymers with linear structure, where x is a small number of
about 10, are oily liquids. These may be compounded with silica aerogel and lithium stearate to produce lubricating greases. When x is a very large number, silicone rubbers
27/38
result. Finally, when the ratio R/Si is lower than 2, combinations of ABC units, crosslinked polymers, are obtained.
The type of organic radical in silicone polymers greatly affects the properties of the resultant products. For example, the methyl silicone polymers with a high R/Si ratio are oily liquids, but the corresponding phenyl silicones of the same chain length are hard, brittle resins, If the methyl group is replaced by an ethyl group a softer, more soluble, and slower-curing product results. Silicones show satisfactory heat resistance at 250°C (482°F); however, at higher temperatures [350°C (662°F)] they undergo extensive degradation and decomposition to low molecular weight products.
Replacement of oxygen with nitrogen in a siloxane leads to the so-called silazanes
which are sensitive to water, decomposing with NH3 evolution. In the search for higher thermal stability numerous polymetallosiloxanes such as silicon-oxygen-aluminum and silicon-oxygen-titanium have been developed:
These materials, however, are very brittle and of very little practical use.
10-16 CHEMICAL RESISTANCE
28/38
Polymers with nonpolar groups such as methyl (CH3) and phenyl (C6H5) are resistant to
polar solvents such as water and ethyl alcohol, but they are usually swollen or dissolved by nonpolar solvents such as gasoline, benzene, and carbon tetrachloride. Furthermore, polymers of more aromatic character are more soluble in aromatic solvents, whereas those of aliphatic character are more soluble in aliphatic solvents. Polymers may be affected by solvents in several ways: dissolution, swelling, permeability, environmental stress cracking, and crazing. The solution process occurs very slowly in two stages: diffusion to produce swelling and then solution.
As the molecular weight of the polymer increases, its solubility or tendency to swell in a particular solvent decreases. Polymers of high molecular weight usually yield solutions of high viscosities. The symmetry of the molecular structure of the polymer also affects the resistance of polymers toward solvents. More crystalline polymers exhibit higher chemical resistance than do less crystalline polymers having the same chemical character. The higher resistance is due to a denser packing of the chain molecules, which makes the penetration of a solvent or other chemical substance in the material more difficult. The degree of crosslinking greatly affects the solubility of polymers, so that even a slight crosslinking insufficient to cause infusibility may make a polymer insoluble. Less effective is the influence of branching, which usually decreases the rate of dissolution, but it does not render a material completely insoluble. The behavior of a heavily branched polymer is similar to that of a slightly crosslinked one, and ¡lis usually very difficult to distinguish between these two types. Crosslinked polymers do not dissolve but only swell if interaction with solvents occurs at all.
Polymers generally have better resistance to attacks by acids and alkalies than metals do; however, they may contain certain vulnerable polar groups that may make them susceptible to attack. Thus alkalies, especially at higher concentrations or higher temperatures, may saponify the ester groupings in cellulose acetate, polyesters, polyvinyl acetate, and similar polymers. Non-oxidizing acids may also hydrolyze these materials in a similar manner. Polyamides (nylons) and polyurethanes having the linkages —NH⋅CO— and —NH⋅COO—, respectively, are also susceptible to attack by strong acids and alkalies. The relative resistance depends on the chain length of the polymethylene groups between the linkages. All polyolefins, PVC, ABS polymers, fluorocarbons, polystyrene, and others have excellent resistance to all acids and alkalies.
10-17 OXIDATION RESISTANCE
29/38
affect melt flow, or elongation, impact strength, electrical properties, surface crazing. and gloss and clarity.
Certain rubbers, especially those that contain a double bond, like natural rubber, styrene—butadiene, neoprene, and acrylonitrile rubber, are very sensitive to oxidation. The attack is enhanced by the presence of ozone in the atmosphere which under normal conditions may range from 0 to 20 parts per 100 million. Ozone (O3), unlike molecular
oxygen (O2), adds directly to a double bond, forming ozonides which further decompose
to peroxides and ketones. In an unstressed rubber a silvery film is formed “on” the surface especially in a humid atmosphere. When rubber is under stress, cracks appear perpendicular to the direction of stress which may lead to failure of the material. Light, especially UV radiation, enhances the action of oxygen, causing deterioration of the physical strength and the phenomenon of crazing, and alligatoring at the surface occurs. When the film is washed away filler particles are exposed which are easily rubbed off. To prevent or to reduce oxidation effects on polymers a number of antioxidants and antiozonants are mixed with the polymer during processing operations. Antioxidants are compounds that inhibit or retard atmospheric oxidation and the effects on a polymer system. There are three main classes of antioxidants used: (1) secondary amine bodies R2NH, which react with chain-propagating radicals that intercept either R⋅ or RO2⋅, (2)
phenolic bodies R(OH)x, and (3) phosphites (RO3)P that decompose peroxide into
non-radical and stable products.
10-18 PERMEABILITY
Closely related to the chemical resistance of polymers is their permeability toward gases and liquids. The passage of gas may take place by simple diffusion through very fine channels between rigid molecular units, as in the case of crystalline, glassy, or highly crosslinked polymers, or by diffusion through viscous materials, as in the case of a rubbery polymer. Diffusion can be enhanced by a solution of gases or liquids in some component of the polymer structure, such as plasticizers. A higher degree of crystallinity, which results in a higher density and also a higher degree of crosslinking, lowers the rates of diffusion of both gases and liquids through the polymer, thereby also improving their chemical resistance. It appears that CO2 has a rate of permeability through most
polymers considerably greater than that of oxygen and still greater than that of nitrogen. This is perhaps due to its ability to be absorbed by materials that permit high rates of water transfer. The average ratio rates between N2: O2: CO2 is 1:4:14.
The absorption of water and its passage through polymer follow general principles: polymers having polar groups show much a higher permeation or absorption rate than nonpolar polymers. Water itself may act as a plasticizer, contributing to the swelling of the polymer and the loosening of its network. The permeability of other solvent molecules under conditions of insolubility of the polymer resembles the mechanism for the permeability of gases, with the added complication of polar effects, as in the case of water transfer. Permeation or permeability rate is the product of the diffusion term and the solubility constant of the gas-liquid in the polymer network,
Permeability rate is a function of many parameters, but the gas permeability coefficient is a basic property of a material independent of specimen geometry.
30/38
the low permeability of a polymer toward oxygen or carbon dioxide PCO2, can make the
material highly permeable toward water and water vapor. For example, polyvinyl alcohol has excellent resistance to gas flow (permeation) but it is a very poor water barrier. In addition, it becomes a poor gas barrier when plasticized with water which causes swelling of the polymer, thereby loosening its structure. Conversely, polyethylene has very good water barrier properties but it is a poor gas barrier. To be a truly good barrier polymer toward the gases the material must have some degree of polarity as provided by nitrile, ester, chlorine, fluorine, or acrylic groups, it should have high chain stiffness, close chain-to-chain packing, crystallinity, and orientation. Note that polyethylene and polypropylene are poor gas barriers but excellent water barriers, whereas polyacrylonitrile is an excellent gas barrier but a poor water barrier. Dimethyl silicone rubber because of its open structure shows extremely high permeability to gases and water. It is used as a membrane oxygenator because of good biocompatibility; however, it is very weak (Table 10-2). The permeability of polymers plays an important part in applications of polymers as films in the packaging industry, for plastic containers, for corrosion-resistant coatings and sheets, for electrical applications, and as membranes in industrial, biological, and waste treatment processes. Permeability of organic coatings to water and oxygen has been extensively studied because of its importance in controlling the corrosion protection of the coating. The presence of particles (fillers and pigments) may affect the permeability of water in a very specific way dependent on the type of particles and pathway mechanisms. The rate of oxygen diffusion through the film was always found to decrease markedly with increasing particle concentration in the film. The rate of transmission of liquid water through glass/polyester laminates and polyester resin castings is a function of resin structure and it always increases with temperature. The effect is a reduction in tensile stress, in flexural strength, and in modulus of elasticity with time when exposed to water for a long time. An additional requirement of high-barrier polymers used in food packaging and beverages is that the taste and odor of the pack aging cannot be affected in any way by interaction with the polymer wall.
10-19 FLAMMABILITY
31/38
Class I forming relatively flame-retardant structures contains either halogen or aromatic groups; all these polymers form char on burning and/or show high thermal stability. High thermal stability is one prerequisite for materials of high fire resistance and low smoke generation. Such materials will yield volatile fuels only under very severe conditions. High aromatic polymers will play an important part in the future of flame-resistant materials because they have very low flammability, generate little smoke during a fire, and do not contain halogens thereby reducing the risk of secondary damage to corrosive gases which may be liberated in afire. No flame-retardant material is required that may adversely affect processing and end-use properties.
Class II compounds do not show much tendency to crosslink or char; but they have a sufficiently close fire-retardant structure so that the addition of flame retardants such as halogen, halogen-antimony, or phosphorus either enhances char formation or inhibits flame generation. Class III represents polymers which are highly flammable and do not char but easily decompose, producing a high proportion of volatile, combustible fragments.
Class III polymers require the addition of flame retardants which are physically compounded with various plastics to meet the specific requirements of flammability. The most important flame retardants are halogenated compounds. phosphorous compounds, metallic oxides, nitrogen compounds, alumina hydrates, and various metallic salts. Flame retardants function as free radicals interrupting melting of the solid polymer and preventing or inhibiting burning of the gases. Triaryl phosphates function very well in the vapor phase while highly chlorinated paraffins can be used to reduce flammability and smoke. Alumina trihydrate is an ideal retardant to be used with fiber-rein forced polymer (FRP) composites. It undergoes endothermic decomposition at 230-300°C (450-570°F) well above molding temperature, to quench a flame and reduce smoke under burning conditions as well as to provide a good arc track resistance in service. Another adverse aspect of the exposure of polymers to fire is the danger of decomposition associated with the emission of toxic fumes and corrosive gases. Even the Class I inflammable polymers such as highly halogenated and highly aromatic polymers may decompose on prolonged exposure to fire, producing highly toxic and corrosive gases.
POLYMER PROCESSING
Polymer processing consists of a series of operations carried Out on polymeric materials to increase their utility. Most polymers are seldom used as pure organic resins, but they are modified and compounded with various additives to obtain desirable properties for particular uses. The process of selection of such additives and their incorporation into a polymer is called compounding. After compounding, (he resultant mix is subjected to a variety of forming and finishing Operations to provide the desired shape, form, and appearance.
10-20 COMPOUNDING
32/38
stabilizers, lubricants, coloring agents, flame retardants, and crosslinking agents, when needed.
Blending of Polymers. Blending is a process of mixing or reacting two or more polymer
resins to obtain a product with improved properties. espcially impact resistance. Blend ing can be accomplished by physical blending, interpolymerization, and graft polymerization. Physical blending is achieved by milling together two incompatible polymers and heating them to above their softening points. On cooling, a two-phase system is formed that consists of a continuous matrix in which the particles of the other constituent are dispersed as spheres, cylinders, rods, or lamellae of varying dimensions. The microstructure and morphology of such a system greatly affect the toughness of the final product. A variety of microstructures are possible, and the dispersed particles may range from 1 to 20 µm, but better properties result with particles from 1 to 5 µm. An example of such a system is the so-called toughened polystyrene (TPS), also called high-impact polystyrene. which consists of a polystyrene matrix with a dispersed rubber phase such as potybutadiene or styrene—butadiene rubber.
Interpolymerization involves the polymerization of a styrene solution of rubber using organic peroxides or azo compounds as catalysts. With increasing polymerization a continuous matrix of polystyrene is formed in which rubber droplets form the dispersed phase. Another two-phase polymer mixture, acrylonitrile-butadiene-styrene, is produced by graft polymerization of butadiene—acrylonitrile or polybutadiene as the rubbery component and styrene-acrylonitrile as the hard, glassy component. High impact strength is achieved by grafting the matrix glassy polymer to the rubber backbone. The dispersed rubber particles are from 0.1 to 0.5 µm.
Plasticizers. Plasticizers are materials that are added to polymeric materials to increase
their plasticity and flexibility. Plasticizers range from solvents with high boiling points to nonvolatile oils and resinous materials. There are three general types of materials used as plasticizers: (1) vegetable oils (nondrying type): (2) monomeric chemicals of high boiling points: and (3) polymeric resinous materials of low molecular weight. The action of plasticizers is considered to be the weakening of the intermolecular forces between macromolecules. This results in greater freedom of movement of the polymeric macromolecules, increasing the flexibility and plasticity of the material but, at the same time reducing its tensile strength and chemical resistance. The effect of plasticizers is to lower the Tg of amorphous polymers and to decrease the degree of crystallinity of
crystalline polymers. The amount of plasticizer added controls the final properties of plastics. For example. PVC is produced as rigid nonplasticized, rigid partially plasticized, and flexible rubbery-type polymer. The most widely used plasticizers are various phthalates.
Fillers and Reinforcing Agents. In order to counteract the weakening effect of the
plasticizer, fillers and various reinforcing agents are added. Fillers improve the strength, impact resistance, heat resistance, dimensional stability, and other similar properties of the polymers. The quantitative aspect of the reinforcement effects of various fillers and fibers is discussed in Chapter 14.
Stabilizers and Other Additives. Stabilizers are added to prevent decomposition or
33/38
10-21 FORMING OPERATIONS
In most forming operations the polymer is subjected to intensive shearing and stressing, usually at molten or nearly molten states. This results in the deformation and orientation of the polymer chains, imparting to the polymer a specific molecular configuration and morphology of the final product. This is particularly strongly marked during spinning, drawing, blowing, and rolling processes, which produce fibers and films. Most forming operations, especially molding, extrusion, and casting, involve the melting or softening of the polymer by heating it to a temperature at which it will flow usually through a narrow nozzle under pressure to fill the cavity of the mold. Under such conditions, most polymeric resins are highly viscous materials having viscosities between 104 and 108 Pa.s
(104 and 109 P), depending on the temperature and pressure. Furthermore, the presence of
fillers and other additives may considerably affect the rheological characteristics of the polymer melts. Usually, polymer melts behave as pseudoplastic materials whose viscosity de creases rapidly with increasing shearing rate. Furthermore, they exhibit viscoelastic behavior that becomes more and more pronounced on gradual cooling of the polymer during molding or extrusion operations. During the polymer flow, the whole polymer chains cannot slide over one another completely, but movement occurs by segmental motion. The movement of the entire chain length is restricted because of numerous entanglements between the chains.
Molding. Molding is our most widely used forming operation; it involves injection
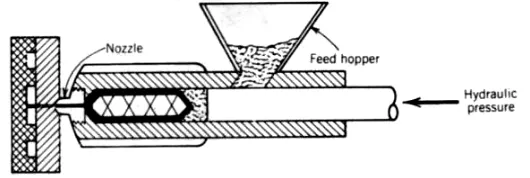
molding, blow molding, compression molding, and transfer molding (see Fig.10-8). Many of the problems that arise during molding, especially injection molding. are caused by rapid changes in the volume and the density of the polymer with pressure and temperature. The increase in density with decreasing temperatures at constant pressure results in considerable shrinkage of the polymer which, in some cases. may result in cracks and imperfections in the molded article. Furthermore, this also gives rise to strains within the body that will adversely affect the mechanical properties and heat resistance of the polymer. To counteract the natural thermal shrinkage occurring on cooling of the polymer from its melt temperature to room temperature, injection molding is usually carried out at as high a pressure as practically feasible. This is because the density of the polymer increases with pressure.
FIGURE 10-8 Diagram of a conventional plunger injection-molding machine. (From Textbook of Polymer Science, 2nd edition, F. W. Billmeyer. Jr., p. 493. Fig. 17-2. Copyright by John Wiley & Sons, Inc., New York, 1971. Reprinted
by permission.)
34/38
For all substances the volume decreases with increasing pressure; hence the compressibility factor is always a positive quantity.
However, the effect of molding pressure on reducing thermal shrinkage is much less in crystalline polymers than in amorphous polymers. This is because crystallization occurring on cooling from the molten state results in much denser material than the amorphous one, causing considerable volume changes. Thus even very high injection pressure cannot prevent excessive shrinkage of the crystalline polymers. The increasing pressure increases the packing density of the polymer chains and favors crystallization at temperatures higher than those for lower molding pressures (Fig. 10-9).
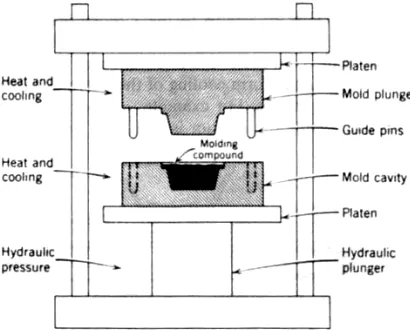
FIGURE 10-9 Diagram of a compression-molding press and mold. (From Textbook of Polymer Science, 2nd edition, F. W. Billmeyer, Jr., p. 492, Fig. 17-1. Copyright O
by John Wiley & Sons, Inc., New York, 1971. Reprinted by permission.)
Another cause of strains in the molded article is the orientation of the polymer molecules during its flow into the mold and through the orifice. On cooling in the cold mold this orientation of the chains becomes frozen; furthermore, a nonuniform cooling of the polymer within the mold also contributes to frozen strains. For example, in thick molding, a stiff outer skin may be formed, while the interior is still fluid. Since the polymer in the interior will subsequently solidify, it will set up stresses in the outer skin. The presence of frozen strains frequently results in the development of a multitude of very small, almost infinitesimal platelike cracks on the surface of the plastic. This phenomenon is called crazing; it may also occur as the result of tensile load acting on the plastic for a long time. Another effect of the presence of frozen strains is a lowering of the distortion temperature of the plastic.
Extrusion. in the extrusion process, the polymer is continuously forced along a screw