Assessment on the Diversity of Parasitoids of
Bagworms (Lepidoptera: Psychidae) in
FELDA Gunung Besout 6, Sungkai, Perak
*Mohd Hanysyam, M.N., *Fauziah, I., **Siti Khairiyah, M.H., *Fairuz, K., *Mohd Rasdi, Z., **Nurul Zfarina, M.Z., *Ismail R. and *Norazliza, R.
*Faculty of Plantation and Agrotechnology, Universiti Teknologi MARA, 40450 Shah Alam, Selangor **Faculty of Applied Sciences, Universiti Teknologi MARA, 40450 Shah Alam, Selangor
E-mail (corresponding authors): [email protected]; [email protected]; [email protected]
Abstract--- Species abundance, diversity, richness, evenness and similarities of parasitoids of bagworms were evaluated at three different localities in an oil palm plantation at FELDA Gunung Besout 6 in Sungkai, Perak. All the three study areas were situated approximately 500m between each other and included the margin and interior part of the oil palm area, and also around the beneficial plants area, which particularly Antigonon leptopus. The abundance, diversity and similarity of insects were varied at all three study sites, which demonstrated the effect of level of disturbances, vegetation diversity and food sources availability. Samplings were conducted for six months starting from August 2011 until January 2012 using Malaise traps. A total of 59 parasitoids, belonging to two orders and seven families were collected from the trap around margin area, whereas a total of 67 insects belonging to two orders and seven families were collected from the trap around the interior area and a total of 68 insects belonging to two orders and six families were collected from the trap around the beneficial plants area. The beneficial plant site has the highest Shannon-Weiner Diversity Index, followed by the interior and the margin site. However, there were no significant differences between the three habitats (p>0.05) through ANOVA analysis. Results of this study showed that even though the level of diversity of parasitoids in the oil palm area of FELDA Gunung Besout 6 was relatively low, there are still some extensive measurement could be carried out for their augmentation and conservation. Factors like food resources, disturbances and anthropogenic effect are still play the main role in conserving the biodiversity and ecosystem in certain area. Hence, the designation of oil palm landscape should be functioned in healthier and more sustainable way in order to provide a wide range of ecosystem services and the conservation of biodiversity.
Keywords-component: diversity, abundance, parasitoids, bagworms, oil palm, FELDA Gunung Besout 6
I. INTRODUCTION
The Malaysian palm oil industry continues to dominate the global supply of the world’s palm oil [1]. Today, the palm oil sector account for nearly seven per cent of Malaysia’s Gross Domestic Product (GDP) and provides more than 1.4 million jobs. Not surprisingly, in 2011, the total oil palm planted area in the whole country has reached 5.0 million hectares, a massive increase of 3.0% against 4.85 million hectares recorded the previous year [2]. It also covered approximately 73% of the total agricultural land and made the oil palm a very promising raw material for renewable energy generation [3].
Bagworm, particularly Metisa plana Walker, is one of the important leaf-eating pests of oil palm in Malaysia and Indonesia. This species of bagworm is capable of being present as outbreak and a damage of 50% would cause high yield losses up to 40-47% or 10 t/ha over two years after a serious infestation [4] - [9]. Crop losses due to the extent of defoliation by a serious bagworm attack, are still inevitable and a moderate defoliation of about 10%-30% may cause a crop loss of about 33%-40% in two years time [8],[10].
Parasitoids are an important group of natural enemies that survive on nectar of beneficial plants as source of food [9], while their life cycles are dependent on their preferred hosts [11]. Bagworm-parasitoid interactions are highly influenced by presence of beneficial plants surrounding the plantation areas [9] and the availability of bagworms as host [12].
In oil palm area of FELDA Gunung Besout 6, chemical insecticides spraying such as trichlorfon, diflubenzuron, cypermethrin or monocrotophos and methamidophos injections have been utilized for bagworms control. Such controls using these chemicals are effective for the beginning of the pest generation as the larvae at the early stage are more susceptible than the next stage [13]. However, the usage of these wide spectrum insecticides are usually would create a long term residue. The residue not only being recognized as the main factor of the recurrent bagworms attack but also is able to extinguish the non-target insect species, especially the natural enemies.
The presence of natural enemies in the study area, such as parasitoids, is doubtful since there were poorly propagated of the beneficial plants. The only species that available was Antigonon leptopus and it was just a few patches around the area. This study was intended to gather information on the availability of parasitoid species that associated with bagworms at three different localities in the oil palm area.
II. MATERIALS AND METHODS
A. Study site
constituted almost 80 oil palm trees. The trees were planted in 1989 and have been yielded at least four times since then.
B. Sampling methods
The study was carried out at three localities, at about 500 m distance from each other. Each locality represented three types of habitat, where insect sampling was conducted: the oil palm margin area; the oil palms interior at a distance of about 250 m from the margin; and the beneficial plants patches (particularly Antigonon leptopus).
Insect sampling was carried out using Malaise traps at every locality in the study area [14]. At all localities, Malaise trapping was carried out once a month. The collecting jars were filled with 70% alcohol and kept open for at least seven to ten days, then cleared from the collected individuals. orders altogether and being stored in vials separately based on their original location of trap. Each specimen were then, pinned, and, once dried, these will be kept indefinitely. Specimens that were too small to pin were mounted on points, on tiny minuten pins. Large and showy insects were mounted in various types of glass-topped display boxes.
Pinning is the best way to preserve hard-bodied insects [15]. The specimens were pinned with a special type of steel pin, well known as insect pin. Insect pin sizes range from 00 to 7, but number 2 and 3 are best for general use. Pinning block were used to obtain uniformity. For moths and butterflies, spreading board was used to spread their wings for easy studied. Insects too small to pin were mounted on a card or point. Points are elongated, triangular pieces of light cardboard or heavy paper, about 8 or 10mm long and 3 or 4mm wide at the base.
Identification of all species of insects was implemented with the help of Entomologist and Science Officers from Strategic Resources Research Centre, MARDI, the Centre of Insect Systematic, Faculty of Science and Technology, UKM Bangi, and also from Department of Biology, Faculty of Applied Sciences, UiTM Shah Alam.
C. Data analysis
A biological community usually has a large number of species with relatively small abundances [16]. When a random sample of individuals is selected and each individual is classified according to species identity, some rare species may not be discovered. This study insect diversity was considered based on the number of species within the sites. The diversity was calculated using the Shannon-Wiener Index [17].
To determine the percent on the insect species similarity, the Jaccard’s Coefficient Index were being used for comparing the similarity and diversity of the insect sample sets [18]. All of the data obtained were encoded and processed by employing Microsoft Office Excel 2007 and the statistical analyses were performed using the Paleontological Statistics (PAST) software programme [14].
D. RESULTSANDDISCUSSION
A. Abundance of all insects in three different habitats
A total 194 individuals of parasitoids that mainly associated with bagworms collected were belonging to only
two orders. There were Hymneoptera and Diptera, which represented by only one family, Tachinidae. The species composition and number of individual parasitoid species collected from the different study sites is shown in Table 1.
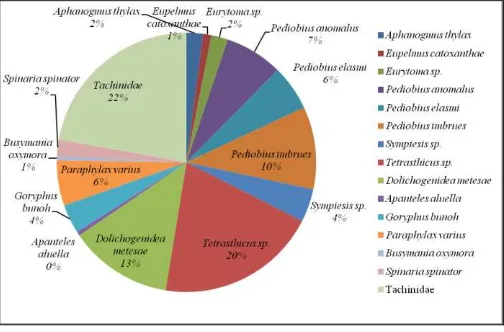
A total of 59 insects belonging to 13 species of Hymenopterans parasitoids and one family of Dipterans parasitoids were collected from margin area in the oil palm plantation. The most abundant family is observed at this site was Tachinidae (20 individuals or 33.9% of the parasitoids collected). Eulophidae constituted 32.2% of the total parasitoids observed at the margin site and was represented by five species. Pediobius anomalus and Tetrastichus sp. were the most abundant species of Eulophidae, followed by Pediobius imbrues, Pediobius elasmi and Sympiesis sp. In addition, 16.95% of the total parasitoids collected belonged to Braconidae, with only Dolichogenidea metesae were found. Ichneumonidae constituted 11.86% from the total of parasitoids recorded and represented by three species, Paraphylax varius, Goryphus bunoh and Spinaria spinator. Only one individual or 1.7% of the total parasitoids were collected from three other Hymenopteran families. There were Ceraphonidae (represented by Aphanogmus thylax), Eupelmidae (represented by Euplemus catoxanthae) and Eurytomidae (represented by Eurytoma sp.).
A total of 67 insects belonging to 11 species of Hymenopterans parasitoids and one family of Dipterans parasitoids were collected from the interior area of oil palm. Eulophidae was the most abundant family collected with 33 individuals, constituted almost 50% of the total parasitoids, with Tetrastichus sp. were being the most abundant species among all parasitoids found (25.37% of total parasitoids), followed by Pediobius imbrues, Pediobius anomalus, Pediobius elasmi and Sympiesis sp. Dipteran family, Tachinidae recorded at least 16% of total parasitoids, followed by Braconidae with 14.93% (represented by Dolichogenidea metesae), Ichneumonidae, 13.43% (represented by two species, Goryphus bunoh and Paraphylax varius) and Ceraphonidea (Aphanogmus thylax) with almost 3%. Euplemidae (Euplemus catoxanthae) and Eurytomidae (Eurytoma sp.) have only one individual found, which constituted 1.5% of the total parasitoids collected.
A total of 68 insects belonging to 13 species of Hymenopterans parasitoids and Dipterans parasitoids were collected from the beneficial plant area in the oil palm plantation. Eulophidae was the dominant family collected (five species, 38 individuals), constituted 55.88% of the total individuals collected. Tetrastichus sp. (Eulophidae) still was the most abundant species found which constituted 23.53% of the total parasitoids found, followed by Pediobius imbrues, Pediobius elasmi, Pediobius anomalus and Sympiesis sp. Tachinidae recorded at least almost 18% of total parasitoids, followed by Ichneumonidae, with 13.24% (represented by four species, Paraphylax varius, Spinaria spinator, Goryphus bunoh and Busymania oxymora), Braconidae, with 8.82% (represented by two species, Dolichogenidea metesae and Apanteles aluella), Eurytomidae, constituted 2.94% (one species recorded, Eurytoma sp.) and Ceraphonidae, with only one individual recorded, Aphanogmus thylax.
Eupelmidae recorded the highest percentage with 47.42% (92 individuals), followed by Dipteran family, Tachinidae with 22.17% (43 individuals), Braconidae (13.4%, 26 individuals), Ichneumonidae (11.86%, 23 individuals), Cerophonidae and Eurytomidae (both constituted 2.06%, four individuals), and Eupelmidae with only 1.03% (two individuals).
Tetratichus sp. from family Eulophidae recorded the highest number in individuals collected in two sites, the
interior and the beneficial plant area, consists of more than 20% of total parasitoids. This species of parasitoid can be found on bagworm, Metisa plana as a primary parasitoid and as a hyperparasitoid on Mahasena corbetti [19]. Most Tetratichus sp. is gregarious larval-pupal endoparasitoid and koinobiont [20] – [23], which it allows the host to continue its development while feeding upon it.
TABLE I. NUMBER OF INDIVIDUALS OF PARASITOIDS COLLECTED FROM THREE DIFFERENT SITES IN THE OIL PALM AREA
Species Family Order Margin Interior Beneficial plant Total
Aphanogmus thylax Ceraphonidae Hymenoptera 1 2 1 4
Eupelmus catoxanthae Eupelmidae Hymenoptera 1 1 - 2
Eurytoma sp. Eurytomidae Hymenoptera 1 1 2 4
Pediobius anomalus Eulophidae Hymenoptera 6 4 4 14
Pediobius elasmi Eulophidae Hymenoptera 2 3 6 11
Pediobius imbrues Eulophidae Hymenoptera 3 6 9 20
Sympiesis sp. Eulophidae Hymenoptera 2 3 3 8
Tetrastichus sp. Eulophidae Hymenoptera 6 17 16 39
Dolichogenidea metesae Braconidae Hymenoptera 10 10 5 25
Apanteles aluella Braconidae Hymenoptera - - 1 1
Goryphus bunoh Ichneumonidae Hymenoptera 2 5 2 7
Paraphylax varius Ichneumonidae Hymenoptera 4 4 3 11
Busymania oxymora Ichneumonidae Hymenoptera - - 1 1
Spinaria spinator Ichneumonidae Hymenoptera 1 - 3 4
Tachinidae Diptera 20 11 12 43
Total individuals 59 67 68 194
Total families 7 7 6
There were other four parasitoid species belonging to Eulophidae family, namely Pediobius imbrues, which constituted 10.31% of total parasitoids, Pediobius anomalus (7.23%), Pediobius elasmi (5.67%) and Sympiesis (4.12%). Pediobius imbrues is mainly found as a primary and hyperparasitoid for bagworm, Metisa plana [19]. This species has broad range of 18 other hosts and acted as an obligate and facultative hyperparasitoids for other Hymenopteran parasitoids [4]. It is also recorded as the most dominant parasitoids found with parasitized bagworms [12]. Due to this fact, it showed that its effectiveness as primary parasitoid was not affected by its hyperparasitic behaviour.
Pediobius anomalus is basically behaving as both primary and hyperparasitoid for two major bagworms, Metisa plana and Pteroma pendula [19]. Pediobius elasmi can be found on other bagworm species, Mahasena corbetti, as primary and hyperparasitoid. As for Sympiesis sp., it can be found on Metisa plana and Mahasena corbetti as a primary parasitoid. Pediobius elasmi and Pediobius imbrues also can be found as hyperparasitoid for two other moth pests, namely Artona catoxantha (Lepidoptera: Zygaenidae) and Parasa lepida (Lepidoptera: Limacodidae) [19].
Family Braconidae was represented by only two species, namely Dolichogenidea metesae and Apanteles aluella. Dolichogenidea metesae was far more abundant in number of individuals than Apanteles aluella in all three sites, consists of 12.89% of total parasitoids collected. Dolichogenidea metesae can be found as primary parasitoid for bagworm. However, its parasitizing activities can be interrupted by Pediobius imbrues, which acted as hyperparasitoid [12]. Apanteles alluella, which only one individual found in the beneficial plant area, was recorded as primary parasitoid for Darna trima (Lepidoptera: Limacodidae), another moth pest species [19].
As for Ichneumonidae, there were four species recorded, namely Paraphylax varius with 5.67% of total parasitoids, Goryphus bunoh (3.61%), Spinaria spinator (2.06%) and Busymania oxymora (0.52%). Paraphylax varius was found as a hyperparasitoid for Dolichogenidea metesae on Metisa plana and some species of nettle caterpillars (Norman et al., 1998). Goryphus bunoh was found as a primary parasitoid for both Metisa plana and Mahasena corbetti but can be a hyperparasitoid for nettle caterpillar species, Setora nitens (Lepidoptera: Cochlidiidae). Another two species of bagworm moths that related to Goryphus bunoh as its host are Amatissa sp. and Dappula sp. (Lepidoptera: Psychidae). Spinaria spinator was only found on nettle caterpillar, Setora nitens, as a primary parasitoid.
Figure 1. The percentage of total parasitoids collected from all three localities in the oil palm area
species recorded. Those families were Ceraphonidae, Eurytomidae and Eupelmidae. Aphanogmus thylax from Cerphonidae consist of 2.06 % or four individuals collected from all three sites. This species can be found on Metisa plana and Mahasena corbetti as a hyperparasitoid [19]. Eurytoma sp. from Eurytomidae also recorded four individuals altogether and can be found on Metisa plana, Mahasena corbetti and other lepidopterans as a hyperparasitoid as well. However, most species of the large and widespread parasitoid genus Eurytoma prefer to attack gall wasps Cynipidae (Hymenoptera) and two other Diptera families, namely Tephritidae (fruite flies) and Cecidomyidae (gall midges or gnats) [24]. As for Eupelmus catoxanthae, only two individuals collected from both matgin and interior sites. This species can be found on Metisa plana and Mahasena corbetti as primary and hyperparasitoid. Other hosts are including moth pest species Artona catoxantha and Parasa lepida.
Tachinidae, the only dipterans parasitoid family recorded comprises 22.17%, making them second most number collected in all three sites, after Hymenopterans family, Eulophidae. Tachinidae is included more than 8,500 described species worldwide and all of them are parasitoids of insects or ohter arthropods, and rank second only to the parasitic Hymenoptera in diversity and ecological importance as insect parasitoids [25]. Therefore, this group has been recognized to be very valuable to humans, because the larval stages are parasites of other insects and many species aid in keeping pest species in balance [15]. Tachnids attack many different groups on insects and although most of them are restricted to certain hosts, a few can develop in a wide variety of hosts. All tachinid species recorded that associated with bagworms can be found on Mahasena corbetti and acted as a primary parasitoid [19].
B. Diversity of all insects in the three different localities The richness of parasitoid species and the abundance in three localities based on diversity and evenness are presented in Table 2. The H’, E’ and R’ values were varied between three different localities in the oil palm area, even though their values are relatively low due to small number of population.
Taxa. S’ Individuals Shannon, H’ Evenness, E’ Margalef, R’
Margin 13 59 2.087 0.620 2.943
Interior 12 67 2.183 0.740 2.616
B/plant 14 68 2.294 0.708 3.081
The higher value of H’ at the beneficial plant site is influenced by the value of (E’ = 0.708) and value of (R’ = 3.081). The high value of diversity in the study site is expected due to the consisted of plant species that play a very
significant role in supporting the sustenance of parasitoids in the oil palm area [9]. This study proved that the practices of establishing the beneficial plants in the oil palm area by the management were able to utilize the parasitoids as the natural enemies of bagworm pests. However, the H’ value is still were uniform in age and size. Hence, this area may not be able to contribute on the sustenance of the parasitoids due to the lack of beneficial plants as food sources. However, it may serve as a support for biological interaction with bagworm pests as hosts for their reproduction and larval development.
As for the margin site, the H’ value (2.087) was the lowest among all three study sites. Nevertheless, the diversity index, H’ value, were still influenced by both E’ (0.62) and R’ (2.943) values. Margin sites were situated at the edge of the oil palm plantation area. This area was adjacent with the main road, management building, factories, stores, houses and others. Hence, it received the most disturbances and anthropogenic effects directly as compared to other two sites. The lack of beneficial plants and oil palm trees also contributed the low level of diversity and species richness in the area.
In general, all of these sites in the oil palm plantation were dealing directly with a lot of factors like the high temperature, low humidity and direct disturbances affect, which lead to the loss of diversity of fauna in the oil palm plantation [26]. These factors were observed and detected in every part of the oil palm studied area. In this case, homogeneous environment, where type of vegetation, rainfall counts, level of humidity and temperature, and soil structure are almost identical in the whole area, is expected to exist. Homogenisation of landscapes due to agricultural intensification is widely recognised as the principal cause of declining farmland wildlife populations [27].
Shannon-Wiener diversity index was then analyzed by using one-way ANOVA in order to compare the significant differences between the three localities. The result showed that there were no significant differences between the three habitats with p>0.05. Therefore, there were no significant values in terms of species distribution in all three sites.
C. Similarity of all parasitoids in three different localities in the oil palm area
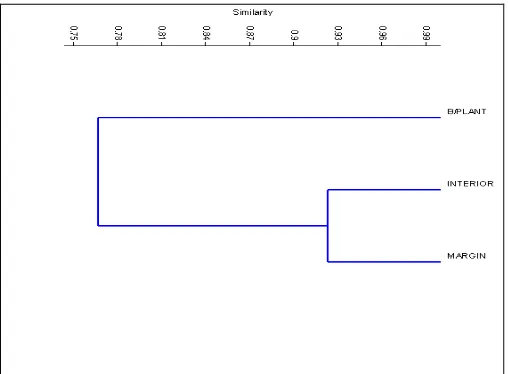
Margin Interior B/plant distances between the three different localities in the oil palm area which influenced by the number of shared parasitoid species.
Figure 2. Dendrogram from a cluster analysis based on presence and absence of each family of non-parasitoids recorded at different localities in the oil palm area, utilizing Jaccard’s Coefficient Index (= similarity index) and the
unweighted-pair groups method (UPGMA).
The interior part of the oil palm plantation area may represent more similar in terms of habitat characteristics and environment with the margin area. The most obvious and important factor is both areas provided less food source for the available parasitoid species in the plantation area.
E. CONCLUSION
Overall, the level of diversity and abundance of parasitoids in the oil palm area of FELDA Gunung Besout 6 is considered low. The impact of oil palm expansion is still severe towards their biodiversity. Taken from the results, the factors like food resources, disturbances and anthropogenic effect are still play the main role in conserving the biodiversity and ecosystem in certain area. Hence, the designation of oil palm landscape should be functioned in healthier and more sustainable way in order to provide a wide range of ecosystem services and the conservation of biodiversity.
ACKNOWLEDGEMENT
Special appreciation to Mr. Norkhaidi Harun, former manager of FELDA Gunung Besout 6 and Mr. Azrulizam Ab. Hamid, assistant manager, for the permission and supports during the field work. Special thanks also to Mr. Jayprakash Pertabai from MARDI and Mr. Roslan Abd. Aziz from UKM, for their assistances in identified the insect species. Thanks to all academic and admin staffs at FPA and FSG for their supports and cooperation. This project was totally funded by Excellence Funds, Universiti Teknologi MARA, 600-RMI/ST/DANA 5/3/Dst (476/2011).
REFERENCES
[1] Craven, C. (2011). The Honduran palm oil industry: Employing lessons from Malaysia in the search for economically and environmentally sustainable energy solutions. Energy Policy 39, pp. 6943-6950. [2] MPOB (2012). Overview of the Malaysian Oil Palm Industry 2011.
Retrieved in Novermber 2012 from
http://bepi.mpob.gov.my/images/overview/Overview_of_Industry_2011 pdf
[3] Ng, W.P.Q., Lam, H. L., Ng., F.Y., Mustafa, K. and Lim, J.H.E. (2012). Waste-to-wealth: green potential from palm biomass in Malaysia. Journal of Cleaner Production 34, pp. 57-65.
[4] Basri, M.W., Norman, K. and Hamdan, A.B. (1995). Natural enemies of bagworm, Metisa plana Walker (Lepidoptera: Psychidae) and their impact on host population regulation . Crop Protection 14(8), pp. 637-645.
[5] Ang, B.N. (2000). Causes of outbreak and failure to control bagworms in oil palm. AA Research News, pp. 1-4.
[6] Mohd Basri, W. and Norman, K. (2002). Cassia cobanensis as a beneficial plant for sustenance of parasitoids in bagworm control. MPOB Information Series 149, p. 2. plana Wlk (Lepidoptera: Psychidae) on young oil palm in a smallholder plantation. Journal of Asia-Pacific Entomology 9(3), pp. 281-285. [9] Norman, K. and Mohd Basri, W. (2010). Interactions of the bagworm,
Pteroma pendula (Lepidoptera: Psychidae), and its natural enemies in an oil palm plantation in Perak . Journal of Oil Palm Research 22, pp. 758-764.
[10] Norman, K., Siti Nurulhidayah, A., Othman, A. and Mohd Basri, M. (2010). Pheromone mass trapping of bagworm moths, Metisa plana
Walker (Lepidoptera: Psychidae), for its control in mature oil plams in Perak, Malaysia . Journal of Asia-Pacific Entomology 13, pp. 101-106. [11] Nor Ahya, M., Rita, M. and Nur Azura, A. (2012). Relationship between
bagworm Pteroma pendula Joannis (Lepidoptera: Psychidae) populations, parasitoids and weather parameters in oil palm plantation. Journal of Agricultural Science 4(12), pp. 13-17.
[12] Cheong, Y.L., Sajap, A.P., Hafidzi, M.N., Omar, D. and Abood, F. (2010). Outbreaks of bagworms and their natural enemies in an oil palm,
Elaeis guineensis, plantation at hutan Melintang, Perak, Malaysia. Journal of Entomology 7(3), pp. 141-151.
[13] Siti Ramlah, A.A., Norman, K., Mohd. Basri, W., Mohd Najib, A., Mohd Mazmira, M.M. and Ahmad Khushairi, D. (2007). Sistem Pengurusan Perosak Bersepadau bagi Kawalan Ulat Bungkus di Ladang Sawit. Lembaga Minyak Sawit Malaysia (MPOB), p. 28.
[14] Szinicz, G., Martin, K. and Sauerborn, J. (2005). Abundance of selected insect species in natural and agricultural habitats of a tropical upland (Leyte, Philippines) Agriculture, Ecosystem and Environment 111, pp. 104-110.
[15] Triplehorn, C.A. and Johnson, N.F. (2005). Borror and Delong’s Introduction to the Study of Insects 7th Edition. Brooks/Cole, Cengage Learning, pp. 745-778.
[16] Chao, A. and Shen, T.J. (2003). Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environmental and Ecological Statistics 10, pp. 429-443.
[17] Joshi, P.C., Kumar, K. and Arya, M. ( 2008). Assessment of insect diversity along on altitudinal gradient in Pinderi forests of Western Himalaya, India . Journal of Asia-Pacific Entomology 11, pp. 5-11. [18] Idris, A.B., Hanidah, J., Gonzaga, A.D. and Nur Azura, A. (2003).
Diversity, abundance, species composition and similarity of genus
Xanthopimpla (Ichneumonidae: Pimplinae) in logged and fragmented forests of the Langat Basin in Selangor, Malaysia. Journal Asia-Pacific Entomology 6(1), pp. 55-62.
[19] Norman, K., Mohd Basri, W. and Zulkefli, M. (1998). Handbook of Common Parasitoids and Predators Associated with Bagworms and Nettle Caterpillars in Oil Palm Plantations. Institut Penyelidikan Minyak Kelapa Sawit Malaysia (PORIM), p. 29.
fruit fly species to Tetrasthicus giffardii Silvestri (Hymenoptera: Eulophidae). Biological Control 39, pp. 262-271.
[21] Liu, H., Bauer, L.S., Miller, D.L., Zhao, T., Gao, R., Song, L., Luan, Q., Jin, R. and Gao, C. (2007). Seasonal abundance of Agrilus planipennis
(Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi
(Hymenoptera: Eulophidae) in China. Biological Control 42, pp. 61-71. [22] Abell, K.J., Duan, J.J., Bauer, L., Lelito, J.P. and Van Driesche, R.G. (2012). The effect of bark thickness on host partitioning between
Tetrastichus planipennisi (Hymen: Eulophidae) and Atanycolus spp. (Hymen: Braconidae), two parasitoids of emerald ash borer (Coleop: Buprestidae). Biological Control63, 320-325.
[23] Yang, S. Duan, J.J., Lelito, J. and Van Driesche, R. (2012). Multiparasitism by Tetrastichus planipennisi (Hymenoptera: Eulophidae) and Spathius agrili (Hymenoptera: Braconidae): Implication for biological control of the emerald ash borer (Coleoptera: Buprestidae). Biological Control.
[24] Mena-Correa, J., Sivinski, J., Anzures-Dadda, A., Ramírez-Romero, R., Gates, M. and Aluja, M. (2010). Consideration of Eurytoma sivinskii
Gates and Grissell, a eurytomid (Hyemnoptera) with unusual foraging behaviors, as a bilogical control agent of tephritid (Diptera) fruit flies. Biological Control 53, pp. 9-17.
[25] Jang, S.A. and Park, C.G. (2010). Gymnosoma rotundatum (Diptera: Tachinidae) attracted to the aggregation pheromone of Plautia stali
(Hemiptera: Pentatomidae). Journal of Asia-Pacific Entomology 13, pp. 73-75.
[26] Ewers, R.M., Fayle, T.M., Foster, W.A., Snaddon, J.L. and Turner, E.C. (2011). The impact of oil palm expansion on environmental change: Putting conservation research in context. Environmental Impact on Biofuels 1, pp. 579-586.
[27] Reid, N., McDonald, R.A. and Montgomery, W.I. (2010).