L
Journal of Experimental Marine Biology and Ecology 248 (2000) 151–161
www.elsevier.nl / locate / jembe
Delayed meiosis and polar body release in eggs of triploid
Pacific oysters, Crassostrea gigas, in relation to tetraploid
production
a ,1 b ,* c
Benoit Eudeline , Standish K. Allen Jr. , Ximing Guo
a
Universite de Caen, Laboratoire de Biologie et Biotechnologies Marine, Esplanade de la Paix, 14032 Caen Cedex, France
b
College of William and Mary, Virginia Institute of Marine Science,
Aquaculture Genetics and Breeding Technology Center, Gloucester Point, VA 23062-1346, USA
c
Rutgers University, Haskin Shellfish Research Laboratory, 6959 Miller Avenue, Port Norris, NJ 08349,
USA
Received 25 April 1999; received in revised form 20 May 1999; accepted 14 January 2000
Abstract
The dynamics of polar body release are important for creating polyploid shellfish. For producing triploids, these dynamics concern meiosis in diploid eggs and are well understood. For creating tetraploids, eggs from triploids are employed and the dynamics, variation, and environmental influences upon polar body release are less studied. We investigated the effects of several agents on the timing of 50% first polar body (PB1) release in eggs of triploids. PB1 release is generally slower in triploid eggs than diploid ones at 268C. Lowering the temperature (from 26 to 198C) had a marked effect on timing of 50% PB1 in both diploid and triploid eggs. While lower temperature merely slowed development in diploid eggs, it nearly halted it in triploid eggs. At any temperature, the variability in 50% PB1 release was much higher in triploid eggs than diploid ones; this variation occurred both within eggs from individual females and among eggs from different females. The amount of time eggs remain in seawater between the time they are stripped and fertilized (or time of hydration) also affected rate of meiosis. In triploid eggs, the average time necessary for the expulsion of 50% PB1 was 23 min post-fertilization (PF) for 75 min of hydration versus 29 min PF for 35 min. However, increasing the time of hydration had no effect on the variability in the timing among females. Serotonin also had no effect on the dynamics of polar body release in triploids. Variability among triploid females in timing of meiosis cannot be
*Corresponding author.
E-mail addresses: beudeline@aol.com (B. Eudeline), ska@vims.edu (S.K. Allen Jr.), xguo@hsrl.rutgers.edu (X. Guo)
1
Present address: Whiskey Creek Oyster Farm, 3395 Bayshore Road, Tillamook, OR 97141, USA. 0022-0981 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
improved with any treatments we tried. Consequently we recommend that treatments of triploid
eggs to produce tetraploids incorporate a single female at a time. 2000 Elsevier Science B.V.
All rights reserved.
Keywords: Crassostrea gigas; Triploid; Tetraploid; Meiosis; Polar body; Flow cytometry
1. Introduction
Technology for chromosome set manipulation in shellfish rests on the principle of manipulation of early developmental events, especially polar body release. Since the earliest reports of polyploidy in shellfish (Stanley et al., 1981), the method of choice has been chemical inhibition of polar bodies, specifically the second polar body to make triploids. The most common chemical treatment is with cytochalasin B (CB) which has, seemingly, the right balance between poison and cure — that is, between killing embryos and creating triploids effectively. Recently, 6-dimethylaminopurine has re-ceived much attention as a replacement chemical to inhibit PB extrusion (Desrosiers et al., 1993; Gerard et al., 1994; Guoxiong and Beaumont, 1996; Nell et al., 1996). Central to the effective use of CB or other chemical inhibitors is understanding early developmental events immediately after fertilization, a relatively simple event to visualize in real time or to document with only a microscope. Still, chemical treatments are onerous because they can be hazardous to the operator and necessarily entail mortality in the non-perfectly dividing eggs.
With the production of tetraploids (Guo and Allen, 1994b), creation of high quality, healthy batches of triploids (and perhaps those of higher ploidies) becomes feasible (Guo et al., 1996). However, the process of creating tetraploids is itself complicated and requires CB (or another inhibitor) to effect polar body inhibition. Specifically, the
process of Guo and Allen (US patent [90976678) requires that eggs from triploids be
fertilized with haploid (from diploid males) sperm, followed by the inhibition of the first polar body. As with the early triploid technology, this method requires working knowledge of the timing of polar body release in eggs of triploids.
Almost nothing is known of meiosis in triploid eggs. Based on some studies by us in the past, it appears that meiosis proceeds normally with the segregation of chromosomes
to each pole, but with the third chromosome segregating at random to yield |1.5n
reduced gametes (Guo and Allen, 1994a). Several lines of evidence corroborate this. Sperm have been shown to have a mean DNA content of 1.5n based on flow cytometric
studies (also see Akashige, 1990). Embryos produced from 3n32n matings have about
25 chromosomes, in both reciprocals. And, embryos from 3n33n matings have a
chromosome count of about 29, one shy of triploid, on average. Recently, Que et al. (1997) demonstrated chromosome segregations in triploid eggs, cytogenetically.
2. Materials and methods
2.1. Brood stock
Triploid Pacific oysters used in this study were 2 years old, and produced by blocking the release of the second polar body (PB2) with CB (Allen et al., 1989). Ploidy was confirmed in all individuals by flow cytometry prior to spawning. Diploid Pacific oysters used in this study were 2 years old and came from a randomly mated population reared in Willapa Bay (Washington State, USA). Both diploid and triploid brood stock were kept in a sea water pond (about 238C) and transferred in 10 000-l conditioning tanks for at least 45 days before spawning.
2.2. Preparation of gametes
Certified triploids were selected, opened, sexed under a microscope and separated by sex to prevent any accidental fertilization of eggs. All surfaces in contact with brood stock were cleaned with diluted bleach. Gametes were obtained by strip spawning.
For females, eggs from individual spawners were removed without adding water, and seawater was added only when all females from an experiment had been stripped (so called dry stripping; Allen and Bushek, 1992). Eggs were separated from cellular debris
by passing them through an 80-mm Nytex screen and held on a 25-mm screen, then
resuspended in 1 mm filtered sea water at 258C and about 30 to 32 ppt salinity. Eggs
were counted by Sedgwick–Rafter chamber using appropriate dilutions. After counting, the eggs were held in seawater at 258C for at least 35 min depending on the experiment. We define the period of time that the eggs remain in seawater before fertilization as ‘hydration’ time (Supan et al., 1996). In this period, germinal vesicle breakdown often occurs in Pacific oyster eggs, and chromosomes progress from prophase of meiosis I to metaphase. Uniform hydration time serves to ‘synchronize’ eggs before fertilization (Allen et al., 1989; Allen and Bushek, 1992). This may be especially important for stripped eggs. After the appropriate period of hydration, two million eggs were divided into two 1-l aliquots for a final concentration of one million eggs per litre. This was done for pools of eggs from both diploids and triploids, yielding a total of four groups.
Sperm was obtained from three males, but only a portion of the gonad was stripped at each experiment (the same day). Afterwards, the top valve was replaced, and partially
stripped males were stored, wrapped in moist paper towel at 48C until the next
fertilization. Allen and Bushek (1992) pointed out that no reduction in sperm viability resulted when males were refrigerated for 24 h. Males were stripped separately 30 min
before fertilization and sperm sieved through a 25-mm screen and resuspended in 1mm
FSW. Just before fertilization, equal aliquots from each sperm suspension were pooled, and the mix used to fertilize eggs. In this way, each replicate was fertilized with the same genetic source of sperm, without risk of loss of sperm activity.
2.3. Fertilization and timing of meiosis
of early development. Aliquots of eggs, divided as above, were fertilized using enough
sperm to obtain |10 spermatozoa per egg under the microscope. For all experiments,
eggs were sampled every 5 min to monitor the kinetics of first polar body extrusion with a light microscope. Fifty eggs were examined to determine the timing of first polar body (PB1) release and the results analyzed by comparing the time necessary to obtain 50% of PB1 extrusion in the eggs for each individual.
2.4. Design of experiments
We compared timing of meiosis in diploid versus triploid eggs under three experimen-tal conditions: temperature, time of hydration, and serotonin treatment.
2.4.1. Rate of meiosis at 19 and 268C
Eggs were hydrated for at least 1 h prior to fertilization. Three replicates using a different female for each replicate and a common pool of sperm were made; fertilized eggs were incubated either at 198C or 268C. After preliminary analysis of the timing of meiosis in these three replications, it was apparent that variability was high among
triploids. We therefore performed a number of additional replicates at 268C to better
describe the variance in meiotic rate in triploids. For these additional replicates (24 diploids and 30 triploids) only the time for 50% PB1 was determined.
2.4.2. Time of hydration
The second experiment examined the effects of holding newly spawned eggs in seawater (hydration) for differing lengths of time. This experiment was accomplished on five triploid females where the eggs from each individual were separated into two
batches and hydrated for either 35 or 75 min in 1 mm FSW at 258C. For these
experiments, we did not examine diploid controls, concentrating instead on whether hydration has any effect on early timing of early meiotic events in eggs of triploids.
2.4.3. Serotonin
The last experiment examined the effects of serotonin on the timing of meiosis. Serotonin was added to the eggs just after their removal from the gonad for the whole
time of hydration (35 min) at 258C, at a final concentration of 10 mM (Osanai and
Kuraishi, 1988). Four triploid and three diploid females were used, and the eggs of each female were separated in two batches, either treated or untreated with serotonin.
2.5. Data analysis
Most of the data were analyzed with the computer software SYSTAT (Systat, Inc., Evanston, IL, USA). Concerning the effects of temperature on the timing of meiosis in diploids and triploids, the data obtained for each replicate were transformed with a logit function, and analyzed by linear regression. The slopes obtained (the speed of release of
PB1) were compared by covariance analysis, with P,0.05 considered significant. For
different. To compare CVs, of the time to 50% PB1 extrusion, we followed Sokal and Braumann (1980) for significance tests on CVs. Also, the time necessary to 50% PB1 release was compared by two-way t-test. The effects of hydration duration and serotonin on meiotic events were analyzed by a paired t-test.
3. Results
3.1. Effects of temperature on meiotic maturation
Meiosis resumed in 97% of diploid oocytes after insemination at 198C and 99% at
268C as indicated by advanced cell division after 2 h of incubation. In triploids, about
75% at 198C and 95% at 268C of the eggs developed.
Higher temperature accelerated polar body release in both diploid and triploid eggs. For the three diploid replicates, the average time to 50% PB1 expulsion was 13.7 min
post-fertilization (PF) at 268C versus 24.7 min PF at 198C. In triploids, 50% PB1
occurred 37 min PF at 268C while at 198C, only 27% of PB1 were expelled after 65 min PF (Fig. 1). PB1 release was significantly faster in diploids at both 268C and 198C, compared to triploids (P,0.05).
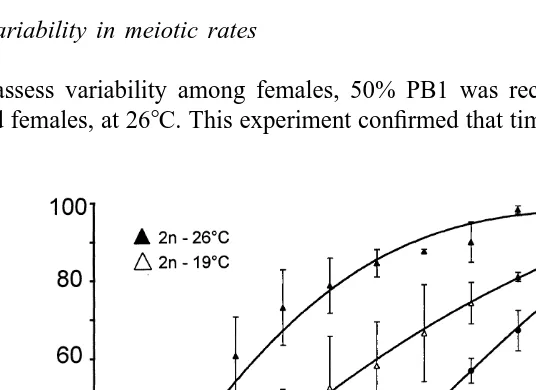
3.2. Variability in meiotic rates
To assess variability among females, 50% PB1 was recorded in 24 diploid and 30 triploid females, at 268C. This experiment confirmed that timing of meiosis in triploids is
Fig. 1. Timing of first polar body (PB1) extrusion in diploid (triangles) and triploid (circles) eggs of
Table 1
Summary of timing of meiosis I (time to 50% polar body 1 extrusion) in eggs from triploid and diploid C.
gigas at 268C
n Average time (min) CV (%)
2n 24 19 13.8
3n 30 33 16.8
significantly delayed (P,0.001) compared to diploids. The average time for 50% PB1
expulsion was 19 min in diploids versus 33 min in triploids (Table 1). The contrast in meiotic rates between diploids and triploids is shown in Fig. 2. All the diploids had expelled 50% PB1 in times ranging from 12 to 23.5 min PF while during the same duration, only 7% (2 of 30) of the triploid females had reached the same stage. It took from 21 to 44.5 min for 50% PB1 expulsion in triploids. Furthermore, variance in timing was greater in triploids than diploids (Table 1). The coefficient of variation of triploids (16.8) was significantly larger than that of diploids (13.8) (P,0.05).
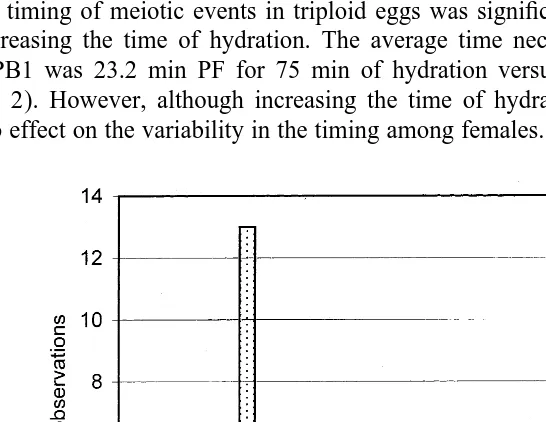
3.3. Effects of hydration on meiotic maturation
The timing of meiotic events in triploid eggs was significantly accelerated (P,0.01) by increasing the time of hydration. The average time necessary for the expulsion of 50% PB1 was 23.2 min PF for 75 min of hydration versus 29.3 min PF for 35 min (Table 2). However, although increasing the time of hydration accelerated meiosis, it had no effect on the variability in the timing among females. In fact for triploid eggs, the
Table 2
Summary of timing of meiosis I (time to 50% polar body 1 extrusion) in eggs from triploid C. gigas as a function of time of hydration of eggs in sea water
Group Time of hydration
coefficient of variation calculated for 75 min hydration (CV526.1) is higher than that of 35 min hydration (CV519.7), but these were not significantly different.
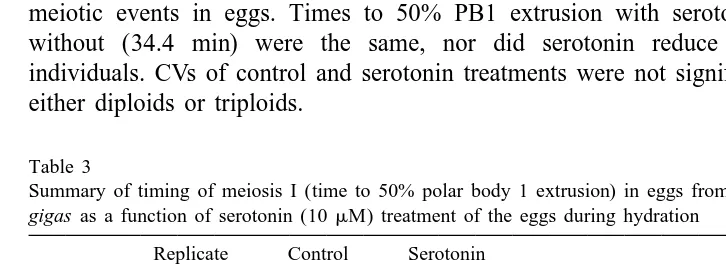
3.4. Serotonin treatments
Serotonin is sometimes used in hatcheries to induce spawning and activate gametes in diploids. We tested this chemical for its potential effect on synchrony and rate of meiotic events in eggs from diploids and triploids. Eggs from two of the three diploid females were apparently slow (Table 3), compared to the females observed in previous experiments (e.g. Table 1). The average time for 50% PB1 for the three diploid females in the serotonin experiment was 28.7 min. However, these same ‘slow’ eggs, in the presence of serotonin, released their polar bodies significantly faster (P,0.05): 20 min
to 50% PB1. In triploids (n54), serotonin was ineffective in changing the timing of
meiotic events in eggs. Times to 50% PB1 extrusion with serotonin (34.5 min) or without (34.4 min) were the same, nor did serotonin reduce variability among individuals. CVs of control and serotonin treatments were not significantly different in either diploids or triploids.
Table 3
Summary of timing of meiosis I (time to 50% polar body 1 extrusion) in eggs from triploid and diploid C.
4. Discussion
4.1. Effects of temperature on meiosis rates
Temperature is one of the most important parameters modifying the timing of meiotic events (Lu, 1986; Downing and Allen, 1987; Allen et al., 1989). Rate of meiosis is certainly as important for making tetraploids as it is for making triploids, especially because of the unusual nature of the polar body manipulation involved. In a triploid egg, inhibition of PB1 causes the reformation of the egg as a hexaploid cell (30 sets of bivalents). According to the hypothesis of Guo and Allen (1994b), a tetraploid results when these 30 sets of bivalents align perfectly along the metaphase plate prior to metaphase II. At metaphase II, the bivalents segregate faithfully into the two daughter cells so that the egg and PB2 both contain 30 chromosomes. When the chromosomes
from the sperm (n510) combine with the 30 remaining in the egg, a tetraploid embryo
results. Unfortunately, the perfect alignment of the 30 sets of bivalent is uncommon. Perfect alignment and subsequent segregation — so-called ‘united bipolar’ segregation — accounts for only 7% of divisions in PB1 inhibited diploid eggs (Guo et al., 1992). Que et al. (1997) confirmed ‘united bipolar’ segregation in triploid eggs by observing
segregation patterns in 3n32n embryos where PB1 had been inhibited. They also noted
that ‘united bipolar’ divisions were more frequent (about 12%) in triploids than those observed by Guo et al. (1992) in diploid eggs. Differences in temperature may affect this
percentage. For example, lower temperatures (such as 198C) may allow a higher
frequency of ‘united bipolar’ alignments, thereby increasing the proportion of tetraploids produced (Guo et al., 1992). Our data confirm that decreasing the temperature leads to a general slowing of meiosis in eggs from diploids and triploids alike. Triploid eggs seem to be more sensitive to temperature change than diploid ones. For example, while meiosis was relatively normal, albeit slower at 198C, in diploid eggs, meiosis in triploids was nearly halted. It was certainly too slow for PB inhibitions and therefore in-appropriate for producing tetraploids. Even if decreasing the temperature increased the percentage of tetraploids, this advantage would be erased by the need to treat the eggs for a much longer period, probably leading to higher larval mortality.
Before these experiments, timing of meiotic events in triploid eggs was unexplored. We show clearly that development of eggs from triploids is delayed compared to diploids in C. gigas. Kawamura (1994) reported similar findings in silkworms (Bombyx mori ): tetraploid spermatocytes needed a longer time to complete the first meiotic division than diploid ones. In the same way, the total larval cycle, from egg deposition to adult emergence, was longer in tetraploids, likely caused by the slower speed of somatic cell cycles (Kawamura, 1994).
4.2. Variability in meiotic rates
be quite effective because the meiotic events are quite predictable and similar among females. However, given the high variability in meiotic events among triploid females, it would be quite difficult, if not impossible, to develop a general technique of inducing tetraploids from pools of triploid eggs. The variability in meiotic events among triploid females could be explained by the diversity of synapsis and segregation patterns previously observed in triploids (Guo and Allen, 1994a). Guo and Allen have shown that although trivalents was clearly the predominant form of synapsed chromosomes in eggs from triploid Pacific oysters, incomplete synapsis was frequent, with a mixture of tri-, bi-and univalents. Depending on the synapsing pattern, we can easily imagine that the timing of meiotic events PF could be variously affected among females.
4.3. Hydration duration
What, if anything, can be done to synchronize eggs for chromosome set manipulation? Hydration (also called ‘soak time’ by Allen and Bushek, 1992) is important for realizing synchronization of the eggs blocked in prophase I. Allen et al. (1989) recommended at
least 30 min of hydration in 258C sea water to increase the synchrony of the eggs and
allow germinal vesicle breakdown (GVBD), a sign that meiosis is preparing to resume. Eggs then remain blocked at metaphase I until fertilization.
Our experiments have shown that increasing the duration of hydration in triploids could accelerate timing of meiotic events, but not reduce the variability in timing of 50% PB1 release among females. Observations made during these trials (data not presented) have shown that even after 35 min hydration, most of the eggs from triploids were not ready to be fertilized, as evidenced by the lack of GVBD. When hydrated for 35 min, then fertilized, some eggs will resume meiosis immediately whereas others will still have to attain GVBD before completing meiosis. However, with longer hydration times, intra-individual variation is decreased and the overall speed of meiosis is faster. The combined result of these events is to better synchronize PB1 expulsion, and therefore, improve the prospects of producing tetraploids.
4.4. Serotonin treatment
4.5. Implications for induction of tetraploidy
Our data have shown that meiotic events in triploids were significantly delayed and variation among females was high, compared to diploids. Delayed meiosis is not a problem for chromosome set manipulation since adjustments can be made in the timing of treatments. That is, if 50% PB release (either the second one for triploids or the first one for tetraploids) is used as a natural indicator of meiotic readiness, the actual elapsed time is irrelevant. However, variation among females in meiotic rate is a problem. Because of this variability, we reasoned that tetraploid inductions need to be adapted to individual females, as opposed to settling for a general rule of induction as is used for making triploids from diploid females. In other words, combining eggs from different females is unlikely to be an efficient approach to tetraploid induction.
Acknowledgements
This work was supported by a USDA SBIR awarded to Whiskey Creek Oyster Farm. We are grateful to its owner, Lee Hanson, for supporting these experiments. We thank Sue Cudd and other hatchery staff for their logistical support, and Taylor United of Shelton, WA for additional assistance. This is VIMS publication no. 2293. [SS]
References
Akashige, S., 1990. Growth and reproduction of triploid Japanese oyster in Hiroshima Bay. In: Hoshi, M., Yamashita, O. (Eds.), Advances in Invertebrate Reproduction, Vol. 5, Elsevier Science, Amsterdam, pp. 461–468.
Allen, Jr. S.K., Bushek, D., 1992. Large scale production of triploid Crassostrea virginica (Gmelin) using ‘stripped’ gametes. Aquaculture 103, 241–251.
Allen, Jr. S.K., Downing, S.L., Chew, K.K., 1989. Hatchery Manual For Producing Triploid Oysters, University of Washington Press, Seattle, WA, USA.
´
Desrosiers, R.A., Gerard, A., Peignon, J.M., Naciri, Y., Dufresne, L., Morasse, J., Ledu, C., Phelipot, P., ´
Guerrier, P., Dube, F., 1993. A novel method to produce triploids in bivalve molluscs by the use of 6-dimethylaminopurine. J. Exp. Mar. Biol. Ecol 170, 29–43.
Downing, S.L., Allen, Jr. S.K., 1987. Induced triploidy in the Pacific oyster, Crassostrea gigas: optimal treatments with cytochalasin B depend on temperature. Aquaculture 61, 1–15.
Fong, P.P., Ram, J.L., Kyozuke, K., Abdelghani, H., Hardege, J.D., Lessanework, D., 1995. The effect of serotonin in fresh and brackish waters on reproduction in the zebra mussel (Dreissena polymorpha). In: 37th Conference of the International Association for Great Lakes Research and Estuarine Research Federation: Program and Abstracts, IAGLR, Buffalo, NY, USA.
Gerard, A., Naciri, Y., Peignon, J.-M., Ledu, C., Phelipot, P., 1994. Optimization of triploid induction by the use of 6-DMAP for the oyster Crassostrea gigas (Thunberg). Aquat. Fish. Man. 25, 709–719.
Gibbons, A.U., Castagna, M.C., 1985. Responses of the hard clam Mercenaria mercenaria (Linne) to induction of spawning by serotonin. J. Shellfish Res. 5, 65–67.
Guo, X., Allen, Jr. S.K., 1994a. Reproductive potential and genetics of triploid Pacific oysters, Crassostrea
gigas (Thunberg). Biol. Bull. 187, 309–318.
Guo, X., DeBrosse, G.A., Allen, Jr. S.K., 1996. All-triploid Pacific oysters Crassostrea gigas Thunberg) produced by mating tetraploids and diploids. Aquaculture 142, 149–161.
Guo, X., Hershberger, W.K., Cooper, K., Chew, K.K., 1992. Genetic consequences of blocking polar body I with cytochalasin B in fertilized eggs of the Pacific oyster, Crassostrea gigas: II. Segregation of chromosomes. Biol. Bull. 183, 387–393.
Guoxiong, C., Beaumont, A.R., 1996. Tetraploid induction in the mussel Mytilus edulis by application of 6-dimethylaminopurine (6-DMAP) during early development. Tropic Oceanol. 15, 26–30, Chinese with English abstract.
Hirai, S., Kishimoto, T., Koide, S.S., Kanatani, H., 1984. Serotonin induction of spawning and oocyte maturation in Spisula. Biol. Bull. 167, 518.
Hirai, S., Kisimoto, T., Kadam, A.L., Kanatani, H., Koide, S.S., 1988. Induction of spawning and oocyte maturation by 5-hydroxytryptamine in surf clam. J. Exp. Zool. 245, 318–321.
Kawamura, N., 1994. Male meiosis in polyploid silkworms, Bombyx mori L. (Lepidoptera: Bombycidae). Int. J. Insect Morphol. Embryol. 23, 311–317.
Lu, J.K., 1986. The combined effects of salinity and temperature on meiosis and early mitosis of the Pacific oyster (Crassostrea gigas) oocytes, University of Washington, Seattle, M.S. Thesis.
Matsunami, T., Nomura, T., 1982. Induction of spawning by serotonin in the scallop Patinopecten yessoensis (Jay). Mar. Biol. Lett. 3, 353–358.
Nell, J.A., Hand, R.E., Goard, L.J., McAdam, S.P., Maguire, G.B., 1996. Studies on triploid oysters in Australia: evaluation of cytochalasin B and 6-dimethylaminopurine for triploidy induction in Sydney rock oysters Saccostrea commercialis (Iredale and Roughley). Aquacult. Res. 27, 689–698.
Osanai, K., Kuraishi, R., 1988. Response of oocytes to meiosis-inducing agents in pelecypods. Bull. Mar. Biol. Stn. Asamushi, Tohoku Univ. 18 (2), 45–56.
Que, H., Guo, X., Zhang, F., Allen, Jr. S.K., 1997. Chromosome segregation in fertilized eggs from triploid Pacific oysters, Crassostrea gigas (Thunberg), following inhibition of polar body 1. Biol. Bull. 193, 14–19. Sokal, R.R., Braumann, C.A., 1980. Significance tests for coefficients of variation and variability profiles.
System. Zool. 29, 50–66.
Supan, J., Wilson, C.A., Allen, Jr. S.K., 1996. The effect of salinity change on the synchrony of polar body development in fertilized eggs (Crassostrea virginica [Gmelin]). In: 1996 Annual Meeting of the National Shellfisheries Association, Baltimore, MD, April, Abstract.
Stanley, J.G., Allen, Jr. S.K., Hidu, H., 1981. Polyploidy induced in the American oyster Crassostrea virginica with cytochalasin B. Aquaculture 23, 1–10.