Summary We compared the growth of trees produced by micropropagation from nodal stem sections or callus tissue of a 20-year-old silver birch (Betula pendula) tree with that of seedlings; growth was monitored for 17 months in pots fol-lowed by six years in the field. Micropropagated trees from both nodal stem sections and callus tissue grew at a similar rate to seedling trees and no obvious mutant types were observed. However, micropropagated trees were more uniform in height and trunk girth than seedling trees and more than 80% flowered within three years of field planting, whereas only 39% of seedling trees flowered within this time. Micropropagated trees had less bark fissuring, a mature characteristic, than seedling trees.
Keywords: Betula pendula, nodel stem explant, callus.
Introduction
Forest and woodland productivity may be increased signifi-cantly by planting mixtures of clones of superior genotypes in place of seedlings of variable phenotypic performance. How-ever, by the time trees are of sufficient maturity to allow selection of genotypes of demonstrated, superior long-term field performance they are usually difficult to clone by conven-tional methods of vegetative propagation.
Micropropagation offers an alternative approach to clonal propagation of mature trees. Fruit trees produced by micro-propagation of mature trees exhibit juvenile characteristics of rapid vegetative growth and improved potential for conven-tional vegetative propagation (Jones 1994).
Although micropropagation has been applied to a range of forest species including some in the mature form, there are few reports of long-term field trials of micropropropagated trees (Chalupa, 1987; McCown and McCown 1987). We have un-dertaken a field trial of micropropagated silver birch (Betula pendula) trees to determine the long-term effect of micro-propagation on growth of this species. We chose to study silver birch because it is an important forest species for which con-ventional propagation becomes increasingly difficult after the trees are about six years old (Welander 1993). We compared the growth of birch trees produced by micropropagation from
nodal stem sections or callus tissue with that of trees grown from seed.
Materials and methods
Micropropagation
Shoot culture Shoot cultures were initiated from a 20-year-old tree (produced by grafting a shoot from a 40-year-20-year-old tree of seedling origin onto a seedling rootstock) growing at the Institute of Forestry Improvement, Ekebo, Svalöv, Sweden. Dormant one-year-old shoots were cut from the tree in Febru-ary 1985, and stored for 2 weeks at 4 °C. The shoots were cut into 2--4 cm lengths, surface sterilized in 70% ethanol for 1 min and then immersed for 15 min in 10% sodium hypochlorite solution with 0.1% Tween-20 wetting agent followed by three washes with sterile distilled water. Apices, 2--4 mm in length, were dissected from the sterilized shoots and placed in 1 mM L-cysteine overnight. Each apex was then transferred a 25 mm × 150 mm plastic-capped polystyrene tube (Guest Medical Ltd., Shipley, UK) containing 10 ml of culture medium. The culture medium was the N6 medium of Simola (1985) supple-mented with agar (Difco Bacto) at 6 g l−1. The pH of the medium was adjusted to 5.5 with 0.1 N NaOH and autoclaved at 120 °C for 20 min.
After four weeks of culture, 22 out of 100 initial shoot tip explants remained sterile and each of these sterile explants produced one or two shoots, 1--2 cm in length. Proliferation of shoots produced by the initial shoot tip explants was carried out in polystyrene tubes containing 10 ml of Woody Plant Medium (Lloyd and McCown 1980) supplemented with agar (Oxoid No. 3) at 6 g l−1, sucrose at 20 g l−1, benzylaminopurine (BAP) at 0.5 mg l−1, naphthalene acetic acid (NAA) at 0.001 mg l−1, and NaFe EDTA at 40 mg l−1, which was the only source of Fe. The medium was adjust to pH 5.5 before being autoclaved.
Two methods were used to proliferate shoots. In the first method, shoot tips, 2--3 mm in length, were removed and the remaining portions of shoots were cut into sections, 1--2 cm in length, each with two nodes. Each nodal stem section was then placed on culture medium in a culture tube. New shoots
pro-Micropropagation of adult birch trees: production and field
performance
O. P. JONES,
1MARGARETA WELANDER,
2BARBARA J. WALLER
1and M. S. RIDOUT
11
Horticulture Research International, East Malling, West Malling, Kent ME19 6BJ, U.K.
2 Department of Horticulture, Swedish University of Agricultural Sciences, P.O. Box 55, S-230 53, Alnarp, Sweden
Received January 25, 1995
duced from the sections were excised after four weeks and the culture procedure repeated each month for six months. In the second method, shoots were placed singly in culture tubes with the basal 2--3 mm of shoot submerged below the culture medium surface. After four weeks, a mass of callus tissue, approximately 0.5 cm in length and breadth, was produced at the base of each shoot. Each callus mass was excised and transferred to a culture tube. Shoots, 1--3 cm in length, pro-duced from the callus within four weeks were excised and the procedures for callus production followed by shoot regenera-tion were repeated each month for eight months.
Culture conditions Initial shoot tip explants were cultured at 23 °C in a 16-h photoperiod at an irradiance of 33 µmol m−2 s−1 from Thorn cool-white fluorescent tubes. Shoot production from nodal stem sections and callus was carried out at 22 °C in a 16-h photoperiod at an irradiance of 88 µmol m−2 s−1 from Crompton warm-white fluorescent tubes.
Rooting Rooting was carried out under the same culture conditions as described for shoot proliferation. Shoots excised from nodal stem sections or callus following six or eight months of sequential subculture, respectively, were placed singly on 10 ml of rooting medium in a polystyrene tube and cultured for two weeks. The Woody Plant Medium used for shoot proliferation was used as the rooting medium except that the macroelements were reduced to one fifth concentration and indole-3-yl-butyric acid (IBA) at 0.1 mg l-1 was the only growth regulator.
Growth in pots Plantlets were transferred from rooting me-dium to 7-cm diameter pots containing compost composed of a 1/1 (v/v) mixture of peat and perlite (Silvaperl standard, Silvaperl Products Ltd., Harrogate, U.K.) and kept for two weeks in propagating trays covered with transparent lids at 22 °C in a 16-h photoperiod at an irradiance 140 µmol m−2 s−1 from Philips color-84 fluorescent tubes. After two weeks, on July 27, 1987, each plantlet was transferred to a pot containing a 3/1 (v/v) mixture of peat and sand supplemented with 4 g m−2 Osmocote slow-release fertilizer (Scott Ltd., Nottingham, U.K.). The potted plants were grown in a heated greenhouse until November 3, 1987 and then transferred to 27-cm diameter pots containing the 3/1 mix of peat and sand supplemented with Osmocote and grown outdoors until they were planted in the field on December 8, 1988.
Seedlings
Seeds from the same tree from which micropropagation was initiated, were germinated in petri dishes containing a 1/1 (v/v) peat/sand mix supplemented with 0.05% P2O5, and 0.33% Ca CO3 at 25 °C in the dark. Approximately 50% of the seeds germinated within two weeks. The seedlings were transferred to pots containing rooting medium and thereafter subjected to the same culture regimes as the plantlets.
Field trial design
Fifty-eight seedlings and 58 plantlets each of stem nodal sec-tion and callus origin were selected at random from the 105 potted plants of each plant type and planted in a field in three
rows of 58 trees with 2 m spacing within rows and 3 m spacing between rows. The soil was a sandy loam soil with a pH of 5.7--6.3. The two trees at each end of each row were guard trees, and the remaining 54 trees formed 18 contiguous blocks of three trees. The complete experiment therefore comprised a randomized block design with 54 blocks. Trees were allocated to blocks on the basis of their height and girth at planting so that the trees within each block were of similar size. In 1992, alternate trees within each row were removed and the 18 original blocks in each row paired to give nine new blocks, each of three trees, though not necessarily one of each treat-ment. Each tree was supported by a bamboo cane during the first year. Weeds in a 1-m wide strip around the trees were controlled by Parable herbicide (a mixture of diquat and paraquat, ICI Ltd.) and by annual mowing around the trees.
Measurements
Numbers and lengths of shoots of the potted plants in the greenhouse were recorded monthly. Heights, lengths of lateral shoots and girths of potted plants grown outside were recorded after one growing season. Tree heights and stem girths at 30 and 130 cm above soil level were recorded in September or October of each year of the field trial. Flowering was recorded by scoring for the presence of male and female flowers accord-ing to the system: 0 = no flowers; 1 = 1--2 flowers per meter of shoot; 2 = 3--10 flowers per meter of shoot; 3 = more than 10 flowers per meter of shoot. Tree spread in the north--south and east--west directions was recorded in 1991 and 1994. Bark fissuring of trees in the field was estimated from the height from soil level to the uppermost fissure on the trunk and from number of trunk fissures at 30 cm from soil level.
Statistical analysis
Logistic regression analysis (Collett 1991) was used for per-centage of trees with forking of the main trunk and of trees with flowers. All other response variables were subjected to analysis of variance.
Results
Micropropagation
The shoot proliferation procedures resulted in a threefold mul-tiplication of shoots from stem nodal sections each month. Shoots were produced from axillary buds or from callus at the cut ends of the nodal stem sections. Shoot production from callus cultures was more variable than from nodal stem sec-tions. The mean number of shoots produced per callus per subculture was 4.0 ± 2.87. Shoots from callus were of similar length and morphology to shoots from nodal stem sections.
Growth in pots
In the greenhouse, all plants had one shoot and no plants flowered. Initially, mean shoot length of micropropagated plants was less than half that of seedlings but by the end of the year it was almost three quarters that of seedlings. Mean shoot length of micropropagated plants from nodal stem sections did not differ significantly (P < 0.05) from that of plants that originated from callus. Initially, seedling plants had slightly fewer leaves than micropropagated plants, but by the end of the year seedlings had about 50% more leaves than micropropa-gated plants. There was no significant difference (P > 0.05) in leaf number between micropropagated plants from nodal stem sections and callus (Table 1).
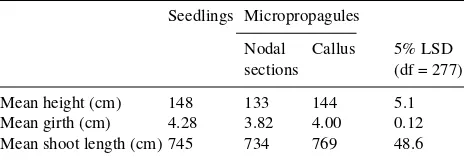
Following transfer to the outdoors, all of the potted trees grew rapidly and produced numerous lateral shoots but there was no flowering. At the end of the growing season, seedlings and micropropagated plants from callus were similar in height, although the former were significantly (P > 0.01) greater in girth. Seedling plants were greater in height and girth than micropropagated plants from nodal stem sections (Table 2).
Growth in the field
All of the micropropagated trees and seedling trees survived
transplanting from pots to the field and there were no obvious mutants among the micropropagated plants. On January 23, 1990, the trees survived winds of up to 78 knots. At transplant-ing, the micropropagated plants and seedlings were similar in general growth habit with most trees having strong apical dominance with straight trunks and numerous lateral branches; however, by 1993, about one sixth of the micropropagated plants and seedling trees had developed forking of the main trunk. There were no significant (P < 0.05) treatment differ-ences with respect to forking. Micropropagated trees and seed-ling trees increased in height by about 1 m per annum and were similar in mean height and girth throughout the duration of the trial (Figures 1 and 2). However, the morphology of seedling trees were much more variable than that of micropropagated trees. By 1994, the variances for heights (m) and girths (cm) at 30 cm were, respectively, 0.242 and 30.11 for seedling trees,
Table 1. Shoot lengths and numbers of leaves of birch seedlings and micropropagated plants from nodal stem sections or callus tissue growing in pots in a greenhouse during 1987.
Seedlings Micropropagules
Nodal Callus LSD df sections (5%)
Mean shoot length (cm)
July 27 3.8 1.3 1.6 0.16 311 September 11 7.9 3.0 3.0 0.33 301 September 25 14.3 7.1 7.2 0.67 301 October 9 28.3 18.7 18.9 1.35 301 November 3 40.7 29.0 28.0 2.02 300
Mean number of leaves
July 27 4.2 5.0 5.1 0.37 310 September 11 10.3 9.3 9.4 0.47 301 September 25 22.9 14.0 13.7 1.06 301 October 9 27.4 18.0 17.4 1.20 301 November 3 32.5 22.2 21.3 1.35 300
Table 2. Mean heights, stem girths at 30 cm above soil level and shoot lengths of birch seedlings and micropropagated plants from nodal stem sections or callus tissue in November 1988 after one growing season in the field in pots.
Seedlings Micropropagules
Nodal Callus 5% LSD sections (df = 277)
Mean height (cm) 148 133 144 5.1 Mean girth (cm) 4.28 3.82 4.00 0.12 Mean shoot length (cm) 745 734 769 48.6
Figure 1. Mean height of birch seedling trees (h) and micropropa-gated trees from nodal sections (s) and callus (n). The vertical bar indicates the LSD for 1994 (df = 51), the LSD for earlier years was smaller.
Figure 2. Mean stem girth at 30 cm (at 130 cm in 1993 and 1994 only) of birch seedling trees (h) and micropropagated trees from nodal
sections (s) and callus (n). The vertical bar indicates the LSD for
callus. The variances for micropropagated trees from nodal stem sections and callus were not significantly different (P <
0.05). In 1991 and 1994, micropropagated trees and seedling trees did not differ significantly (P < 0.05) in mean canopy
spread in the north–south and east–west directions.There was much more extensive fissuring of bark in seedling trees than in micropropagated trees (Figure 3). In September 1994, after six years in the field, the mean number of fissures at 30 cm from ground level for seedling trees was 15.1 compared with 7.2 and 7.9 for trees micropropagated from nodal sections and cal-lus, respectively (LSD at 5% = 1.80, df = 51). Mean height of the highest fissure was 68.6 cm for seedling trees and 29.5 and 34.1 for trees micropropagated from nodal section and callus, respectively (LSD at 5% = 8.35, df = 51).
Flowering
No flowers appeared on any of the trees until 1990 when one flower was recorded on 7% of each of the seedlings and both sets of micropropagated trees. In 1991, 80% or more of the micropropagated trees and 39% of the seedling trees recorded a score of 1 for flowering, and this difference between seedling trees and micropropagated trees was significant (P < 0.001).
All micropropagated trees and most seedling trees flowered by 1993, but intensity of flowering remained significantly greater on micropropagated trees (P < 0.01) than on seedling trees
throughout the trial (Table 3).
Discussion
The micropropagation methods that we used have been ap-plied successfully to a range of adult genotypes of Betula pendula (Welander 1993) and are similar to methods used for
other species ofBetula (Meier-Dinkel 1992).
The comparison between seedlings and micropropagated plants may be confounded by the range of genotypes that comprised the seedling population in contrast to the single genotype that comprised the micropropagated plants; how-ever, it is possible to make some valid conclusions.
Although seedlings are known to grow more vigorously than mature trees (Jones 1994), we found that micropropagated trees from both nodal stem sections and callus had similar rates of stem elongation as seedling trees. The high rate of stem extension of micropropagated trees may have resulted from a process of rejuvenation as a result of culturein vitro. The low
incidence of fissuring in micropropagated trees compared with
Table 3. Flowering of seedling trees and micropropagated birch trees. Mean flowering score was based on a scale of 1 to 3, where 1 represents the least number of flowers per tree.
Seedlings Micropropagated trees
Nodal sections Callus LSD (5%) df
Percentage of trees with flowers
1990 7 7 7
1991 39 80 87
1992 61 98 100
1993 93 100 100 1994 96 100 100
Mean flowering scores of trees with flowers
1990 1.0 1.0 1.0 – 1991 1.0 1.0 1.0 –
incidence of fissuring in micropropagated trees compared with seedling trees may be indicative of micropropagation-induced rejuvenation (Whitmore 1962). Various juvenile morphologi-cal and biochemimorphologi-cal features have also been reported among micropropagated plants from mature trees of Betula species (Brand and Lineberger 1992a, 1992b). However, we found that micropropagated trees flowered sooner than seedlings indicat-ing that not all features of the micropropagated trees were juvenile.
The nodal stem sections appeared to produce axillary shoots from lateral buds and adventitious shoots from callus at the cut ends, whereas the callus cultures appeared to produce only adventitious shoots. Welander (1988) reached similar conclu-sions about the origins of in-vitro-derived shoots of Betula pendula based on anatomical studies. Adventitious shoots may give rise to somaclonal variants (Hammerschlag 1992), how-ever, we observed no evidence of obvious mutant types among the micropropagated trees. On the contrary, the micropropa-gated trees were much less variable than the seedling trees, possibly because the micropropagated trees were more uni-form genetically than the seedling trees. Based on the results of this long-term field trial of micropropagated trees from mature Betula pendula, we conclude that micropropagation can be used to provide uniform trees that have the rapid growth characteristics of seedlings (McCown and McCown 1987).
Acknowledgments
The research described in this paper was funded by the Biotechnology and Biological Science Research Council and the East Malling Re-search Association. The authors thank Mr. W.C.C. Hadlow for assist-ing in collection of data.
References
Brand, M.H. and R.D. Lineberger. 1992a. In vitro rejuvenation of
Betula (Betulaceae): Morphological evaluation. Am. J. Bot. 79:618--625.
Brand, M.H. and R.D. Lineberger. 1992b. In vitro rejuvenation of
Betula (Betulaceae): Biochemical evaluation. Am. J. Bot. 79:625--635.
Chalupa, V. 1987. European hardwoods. In Cell and Tissue Culture in Forestry, Vol. 3. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff. Pub., Dordrecht, Boston, Lancaster, pp 224--246. Collett, D. 1991. Modelling binary data. Chapman and Hall, London. Hammerschlag, F.A. 1992. Somaclonal variation. In Biotechnology of Perennial Fruit Crops. Biotechnology in Agriculture No. 8. Eds. F.A. Hammerschlag and R.E. Litz. CAB Int., pp 35--55.
Jones, O.P. 1994. Physiological change and apparent rejuvenation of temperate fruit trees from micropropagation. In Physiology, Growth and Development of Plants in Culture. Eds. P.J. Lumsden, J.R. Nicholas and W.J. Davies. Kluwer Academic Publishers, Dor-drecht, The Netherlands, pp 323--331.
Lloyd, G. and B. McCown. 1980. Commercially feasible micropropa-gation of mountain laurel Kalmia latifolia by use of shoot tip culture. Proc. Int. Plant. Prop. Soc. 30:421--427.
Meier-Dinkel, A. 1992. Micropropagation of birches (Betula spp.). In
Biotechnology in Agriculture and Forestry, Vol. 8. Hightech and Micropropagation II. Ed. Y.P.S. Bajaj, Springer-Verlag, Berlin, Heidelberg, pp 40--81.
McCown, D.D. and B.H. McCown. 1987. North Americal Hard-woods. In Cell and Tissue Culture in Forestry, Vol. 3. Eds. J.M. Bonga and D.J. Durzan. Martinus Nijhoff Pub., Dordrecht, Boston, Lancaster, pp 247--271.
Simola, L.K. 1985. Propagation of plantlets from leaf callus of Betula pendula f. purpurea. Sci. Hortic. 26:77--85.
Welander, M. 1988. Biochemical and anatomical studies of birch (Betula pendula Roth.) buds exposed to different climatic condi-tions in relation to growth in vitro. In Genetic Manipulation of Woody Plants. Eds. J.W. Hanover and D.E. Keathley. Plenum Pub-lishing Corporation, New York, pp 79--99.
Welander, M. 1993. Micropropagation of birch. In Micropropagation of Woody Plants. Ed. R. Ahuja. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 223--246.