16 Brooks, R.R. et al. (1992) The serpentine vegetation of Goiás State, Brazil, in The Vegetation of Serpentine Soils (Baker, A.J.M. et al., eds), pp. 67–81, Intercept
17 Reeves, R.D. et al. (1995) Abnormal accumulation of trace metals by plants, Mining Environ. Mgt 4–8 September
18 Brooks, R.R. (1977) Copper and cobalt uptake by Haumaniastrum species, Plant Soil 48, 541–545 19 Reeves, R.D. and Brooks, R.R. (1983)
Hyperaccumulation of lead and zinc by two metallophytes from a mining area of Central Europe, Environ. Pollut. Ser. A 31, 277–287 20 Jaffré, T. (1980) Etude Ecologique du Peuplement
Végétal des Sols Dérivés de Roches Untrabasiques en Nouvelle Calédonie, ORSTOM (New Caledonia)
21 Minguzzi, C. and Vergnano, O. (1948) Il contenuto di nichel nelle ceneri di Alyssum bertolonii, Atti Soc. Tosc. Sci. Nat. 55, 49–74
22 Morrey, D.R. et al. (1992) A review of some studies of the serpentine flora of South Africa, in The Vegetation of Serpentine Soils (Baker, A.J.M. et al., eds), pp. 147–157, Intercept
23 Cannon, H.L. (1964) Geochemistry of rocks and related soils and vegetation in the Yellow Cat area, US Geol. Surv. Bull. 1176, 1–127 24 Leblanc, M. et al. The phytomining and
environmental significance of hyperaccumulation of thallium by two plant species, Econ. Geol. (in press)
25 Baumann, A. (1885) Das Verhalten von Zinksalzen gegen Pflanzen und in Böden, Verh. Landwirtsch. Vers. Stn 31, 1–53
Robert Brooks*and Brett Robinson are in Soil Science, College of Sciences, Massey University,
Palmerston North, New Zealand; Michael Chambers is at
Box 13511, Reno, NV 89507, USA; Larry Nicks is at Box 650, Fernley, NV 89408, USA.
*Author for correspondence
(tel +64 6 3569099; fax +64 6 3505632; e-mail [email protected]).
Chemical ecology is the study of chemical interactions between organisms and their environment. By analyzing the chemical traits that mediate interactions among organisms (such as the attraction of mates, dispersal of offspring, defense against enemies and com-petition for resources), researchers have impli-cated an ever-growing catalog of compounds. However, enormous challenges remain in elu-cidating the roles of individual chemicals and determining their evolutionary origins. For ex-ample, many plants produce complex mix-tures of organic compounds that are thought to be important in defense against herbivores, but few experiments have demonstrated the function of the individual components. Gen-etic and molecular methods have great poten-tial for addressing these questions.
Institute for chemical ecology
Research into the basis of the evolutionary forces that shape chemically mediated eco-logical interactions requires an interdiscipli-nary approach. This was the rationale for the new Max Planck Institute for Chemical Ecol-ogy in Jena, Germany. At a molecular level, researchers are seeking to characterize and elucidate the function of individual genes in-volved in the synthesis, storage, detection and metabolism of the compounds that mediate plant–pest interactions. Because plants play a central role in most ecosystems, and because
they have developed a rich set of chemically mediated interactions with the community of heterotrophs that attack them, the chemical ecology of plants is the primary focus for re-search into the molecular and population gen-etics determining chemical traits. By bringing together researchers in ecology, population genetics, biochemistry, entomology, organic synthesis and analytical chemistry, the insti-tute will be able to study the functional basis of chemically mediated ecological interac-tions in an interdisciplinary environment.
Evolutionary history
In 1888, Ernst Stahl, a professor in Jena, noted a pattern of reciprocal adaptation between plants and their insect herbivores1. The disci-pline of chemical ecology expanded through-out this century2, and a landmark paper by Ehrlich and Raven3has shaped the research agenda in recent decades. They suggested a model of stepwise chemical coevolution between plants and insects, and proposed that antagonistic chemical interactions between plants and their natural enemies are primary factors responsible for the adaptive radiation of both plants and herbivorous insects. These historical interactions may be responsible for current patterns, where related plant species have similar secondary chemistry, and closely related insect taxa choose similar host plant species.
Subsequently, the concept of coevolution has been refined to distinguish between pair-wise and diffuse coevolution4. Pairwise co-evolution refers to a reciprocal, stepwise ‘arms race’ between an insect species and its host plant. Diffuse coevolution is more common, whereby several related insect species attack a range of plant species with similar chemical profiles. Although several herbivore and host species can have important impacts on each others’ evolution, tightly coevolving species pairs are probably rare5.
In contrast to theories of chemical coevolu-tion, the role that plant chemistry plays in determining insect host choice has recently been questioned6on the grounds that natural enemies of herbivorous insects might play a predominant part in determining insect host association. According to this view, plant sec-ondary chemistry functions in other physio-logical roles, such as defense against UV-B radiation, drought, or other abiotic stresses. Alternatively, secondary metabolites might function in overflow storage or disposal of waste products from primary metabolism.
Molecular approaches
Questions about the adaptive origin and cur-rent function of plant secondary chemistry can be addressed using methods from molecular biology and evolutionary genetics, particularly via the isolation, characterization and manipu-lation of genes7. Here we illustrate several ap-proaches that use physiological information, natural genetic variation, or molecular ma-nipulations to understand the role and conse-quences of plant secondary chemistry.
Induction experiments
Inducible defenses may provide effective defense against attack by insect herbivores, while avoiding physiological costs of defense8 when herbivory is absent. However, such pre-sumed cost-savings and fitness benefits have not been demonstrated in nature. Ecological consequences of induced plant defenses can be studied using the wound-induced plant hormone jasmonic acid, which causes up-regulation of secondary chemicals in many plant species9. In the native, post-fire annual, Nicotiana attenuata (Fig. 1), the level of toxic nicotine increases after herbivore attack and is internally activated by jasmonic acid. In 745 matched pairs of N. attenuata plants growing in native populations, one member of each pair was treated with jasmonate methyl ester
362
trends in plant science
update
September 1998, Vol. 3, No. 9 Copyright © 1998 Elsevier Science Ltd. All rights reserved. 1360 - 1385/98/$19.00 PII: S1360-1385(98)01296-5
Chemical
to elicit putative defensive compounds10. Life-time production of viable seed was measured in control and treated plants growing in several native populations with different probabilities of attack from herbivores. In populations with intermediate levels of herbivory, induced plants experienced less insect damage and produced significantly more seed than noninduced con-trols. However, when herbivory was low or absent, induced plants had lower fitness than their noninduced counterparts. These results indicate that natural selection favors jasmonate-induced defenses in the face of herbivory. How-ever, investment in such defenses is costly, and reduces plant fitness when herbivory is low. Inducible defenses allow Nicotiana to forgo these costs when defense is unnecessary. In addition to eliciting the production of a large variety of low molecular weight toxins and defensive proteins, herbivore attack also stimu-lates the release of volatile compounds (Fig. 2). These might function as ‘alarm calls’, that at-tract parasitoids and predators of insect herbi-vores, and so allow a plant to recruit the third trophic level for its defense11. The octadecanoid cascade is involved in this release, because structural analogs of jasmonic acid (specifi-cally coronatine and amino acid conjugates of indanones), volicitin (the first low molecular weight insect-specific elicitor) and cellulysin elicit volatiles comparable to those released after herbivore attack9,12,13. These jasmonate analogs can be used to characterize the signal transduction cascades involved in induced defenses. Once the mechanisms responsible for the production, elicitation and release of these volatile signals are known, rigorous examinations of their functional consequences under field conditions will be feasible.
Quantitative genetics
The genetic variation in insect resistance and two defensive traits, trichome density and glu-cosinolate concentration, have been studied in natural populations of Arabidopsis14. Geno-types were replicated in two environments, either with natural herbivores present, or where
natural selection caused by herbivory had been eliminated by insecticide treatment. With this experimental design costs of resistance can be detected if resistant genotypes have higher fit-ness in the presence of herbivores, and if sus-ceptible genotypes are favored when insects are absent. By comparing effects of trichomes and glucosinolates on fitness under natural field conditions, it was possible to identify the components of natural selection exerted by herbivores on these traits. It was apparent that natural enemies favored genotypes with more trichomes and higher concentration of gluco-sinolates, showing that these secondary com-pounds are functionally important in defense against herbivores. In addition, this experi-ment detected significant genetic costs to re-sistance under natural field conditions. Thus, herbivores can exert a selection pressure for higher investment in mechanisms of plant de-fense. However, these investments are costly, and their overall selective value depends on the level of herbivory in particular environments.
QTL mapping
One method for understanding the role and consequences of variation in plant chemistry is mapping of quantitative trait loci (QTL)15. Chromosomal locations of genetically variable loci that influence physiology and secondary chemistry can be determined using linkage maps of molecular markers. When QTLs affect-ing physiological variation map near enzyme-encoding loci, this genetic variation may be caused by coding changes that alter the poly-peptide sequence of enzymes, or by cis-acting regulatory variation at the enzyme locus.
An important question is whether genetic variation for physiological traits is caused by substitutions that alter amino acid sequences of enzymes, or by variation in cis- or trans-acting regulatory sequences. This was addressed by examining quantitative genetic variation in levels of enzyme activity for six glycolytic and four defense enzymes in Arabidopsis16. Some QTLs gave suggestive evidence regarding the causes of quantitative genetic variation. For example, a QTL influencing levels of hexo-kinase activity mapped very near AtHXK1, a hexokinase encoding locus. However, both AtHXK1 alleles in this population have iden-tical cDNA sequences, so activity variation was not caused by amino acid polymorphisms that altered the coding sequence of this en-zyme. Consequently, this QTL must be caused by variation in cis-acting regulatory factors at the AtHXK1 locus, or else at a closely linked regulatory locus.
This study also found that QTLs that influ-ence secondary metabolism had significantly larger effects than QTLs that affect primary metabolism. Although based on only a few enzymes, this trend conforms with an intuitive attitude to physiological and evolutionary
363
trends in plant science
update
September 1998, Vol. 3, No. 9
Fig. 1. Mandua quinquemaculata feeding on Nicotiana attenuata in native populations in southwestern Utah. Feeding by these specialist lepidopteran larvae dramatically induces the jasmonate cascade which, in turn, elicits both direct (e.g. nicotine, proteinase inhibitor induction) and indirect (release of mono- and sesquiterpenes) de-fenses in the plant. The evolution-ary response to herbivory in this plant includes its germination be-havior, which synchronizes ger-mination (with gerger-mination cues found in wood smoke) from long-lived seed banks with the im-mediate post-fire environment, an environment with low herbivore loads and high soil nutrient re-gimes. The high soil nutrient regimes may defray the
resource-Fig. 2. Jasmonic acid, putative jasmonate mimics (coronatine and indanone-isoleucine methyl ester), and volicitin elicit the release of volatiles from plants that may function as ‘alarm call’ defense signals9,12,13
. Volicitin is found in the oral secretions of the beet army-worm13
and coronatin is produced by some Pseudomonas syringae pathogen varieties12 . O
O
O NH
HOOC
O
O NH O
O H3C
H N
O
O H2N
OH O
Jasmonic acid Coronatine Indanone-Ile ME
COOH
HO
pressures. Enzymes of secondary metabolism may experience weaker natural selection or geographically heterogeneous selection press-ures5in comparison with enzymes of primary metabolism, which are essential for growth and survival17.
Molecular evolution
The biosynthesis and regulation of flavonoids is one of the best characterized areas of second-ary metabolism18. These compounds can act as pigments, provide chemical defenses against microorganisms, signal between plants and Agrobacterium or Rhizobium, and also func-tion as a ‘sunscreen’ against UV-B radiafunc-tion. Gene duplication and acquisition of new meta-bolic roles is common in this pathway19. For example, at least three independent evolu-tionary origins of stilbene synthases from chalcone synthases have occurred in different plant families20.
Hanson and colleagues21reported a com-bined genetic and molecular evolutionary study of anthocyanin biosynthesis in maize and teosinte. Teosinte, the ancestor of modern maize, has drab kernels that lack anthocyanin pigments. During the past 7500 years, early farmers selected for colorful, anthocyanin-pigmented maize kernels. Using genetic tester stocks in maize, it was shown that teosinte has functional alleles of all enzyme-encoding loci necessary for pigment biosynthesis. However, although the c1 and r regulatory loci encode
functional proteins, they are not expressed during kernel development, so anthocyanin biosynthesis is not activated in these tissues. Analysis of molecular variation at the c1 locus suggests that early farmers imposed selection for colorful kernels, favoring alleles lacking the Box I ‘gtgtc’ motif at the c1 regulatory locus. This combination of functional and evolutionary analysis provides evidence that differences in coloration between teosinte and maize are caused by selection for naturally occurring variation at regulatory loci, rather than changes in enzyme encoding genes.
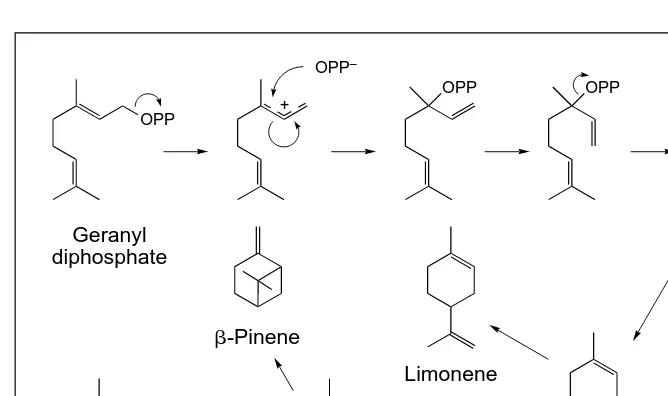
Less is known about other pathways of sec-ondary metabolism, although there has been recent progress in understanding the molecu-lar biology of alkaloid22and terpenoid biosyn-thesis. For example, nearly 30 genes have now been isolated that encode terpene synthases, the enzymes that catalyze formation of the basic carbon skeleton of terpenoids from acyclic prenyl diphosphates. Sequence com-parison and phylogenetic analyses show that all known terpene synthases share a common evo-lutionary origin, but lack strong similarities to other enzymes except the mechanistically simi-lar prenyltransferases23. The expression of cloned terpene synthases has revealed that many of these enzymes have the unusual ability to synthesize multiple products (Fig. 3), which helps to explain why terpene-accumulating plants usually possess a complex mixture of terpenoid metabolites. Mixtures are thought to
enhance the defensive potential of plants by the synergistic action of their constituents and by delaying the acquisition of resistance in herbivores24.
Future prospects for genetic and molecular manipulation
Altered gene expression by genetic engineer-ing or mutagenesis can provide information on physiological mechanisms and the functional role of plant secondary chemistry. There have been several studies on the biochemical gen-etics of plant response to biotic and abiotic stress. For example, overexpression of chitin-ase increchitin-ases resistance to fungal pathogens in Brassica25. Increased concentrations of pro-tease inhibitors increase insect resistance in several plant species26. Phytoalexin deficient pad mutants of Arabidopsis are more suscep-tible to virulent strains of Pseudomonas syrin-gae27. Mutants with alterations in jasmonic acid synthesis or signal transduction have cor-responding changes in resistance to fungus gnats28or Pseudomonas syringae29. Arabid-opsis tt4 mutants deficient in flavonoid bio-synthesis are more susceptible to the damaging effects of UV-B radiation30, demonstrating that these secondary compounds play a role in resistance to abiotic stress.
Few studies have used these methods to ad-dress ecological and evolutionary questions31,32, but the tools are now available to generate mu-tants or transgenic plants with which to study ecologically important traits. The ecological and fitness consequences of these manipu-lations can then be examined in greenhouse or field experiments. Using this carefully tar-geted approach we can begin to address fun-damental questions in chemical ecology, such as the functional role of secondary com-pounds, costs of resistance, or whether plants can gain partial protection against herbivorous insects by attracting predatory insects using volatile compounds under field conditions.
Acknowledgements
Our work is supported by the Max-Planck-Gesellschaft and by grants from the European Union (T.M-O.), US National Science Foundation (T.M-O., I.B.), US Dept of Energy (J.G.), A.W. Mellon Foundation (I.B.) and Deutsche Forschungsgemeinschaft (W.B.). Thanks to J. Bishop, M. Gurganus and J. Kroymann for comments on the manuscript. We thank S. Dix for secretarial assistance.
Thomas Mitchell-Olds*, Jonathan Gershenzon, Ian Baldwin and Wilhelm Boland
Max-Planck-Institut für Chemische Ökologie, Tatzendpromenade 1a, 07745 Jena, Germany
*Author for correspondence
(tel +49 3641 643657; fax +49 3641 643668; e-mail [email protected])
364
trends in plant science
update
September 1998, Vol. 3, No. 9
Fig. 3. Proposed mechanism of limonene synthase, a terpene synthase that produces mul-tiple products as demonstrated by isotopically-sensitive branching experiments33and cDNA cloning34. Several of the postulated reaction intermediates are carbocations (intermediates in which a carbon atom bears a positive charge). These reactive species have a variety of chemical fates and may be responsible for the ability of terpene synthases to make multiple products. The ability to make multiple products may in turn account for the prevalence of mixtures of terpene defensive substances in terpene-accumulating plants, a trait with sig-nificant ecological consequences24. ‘OPP’ indicates a diphosphate group, and curved arrows illustrate electron flow during the reaction.
OPP
Geranyl diphosphate
OPP–
+
OPP OPP
+
Myrcene
+
Limonene
+
b-Pinene
References
1 Stahl, E. (1888) Pflanzen und Schnecken. Eine
biologische studie uber die schutzmittel der pflanzen gegen schneckenfrass, Jenaischen
Zeitschrift Medizin fur Naturwissenschaft und Medizin 22, 557–684
2 Feeny, P. (1992) The evolution of chemical
ecology: Contributions from the study of herbivorous insects, in Herbivores: Their
Interactions with Plant Metabolites (Vol. 2)
(Rosenthal, G.A. and Berenbaum, M.R., eds), pp. 1–44, Academic Press
3 Ehrlich, P.R. and Raven, P.H. (1964) Butterflies
and plants: a study in coevolution, Evolution 18, 586–608
4 Iwao, K. and Rausher, M.D. (1997) Evolution of
plant resistance to multiple herbivores: quantifying diffuse coevolution, Am. Nat. 149, 316–355
5 Thompson, J.N. (1994) The Coevolutionary Process, University of Chicago Press 6 Jermy, T. (1988) Can predation lead to narrow
food specialization in phytophagous insects?
Ecology 69, 902–904
7 Settles, A.M. and Byrne, M. (1998) Opportunities
and challenges grow from Arabidopsis genome sequencing, Genome Res. 8, 83–85
8 Gershenzon, J. (1994) The cost of plant chemical
defense against herbivory: a biochemical perspective, in Insect–Plant Interactions (Vol. 5) (Bernays, E.A., ed.), pp. 105–173, CRC Press
9 Boland, W. et al. (1995) Jasmonic acid and
coronatin induce odor production in plants,
Angew. Chem. Int. 34, 1600–1602 10 Baldwin, I.T. (1998) Jasmonate-induced
responses are costly but benefit plants under attack in native populations, Proc. Natl. Acad.
Sci. U. S. A. 95, 8113–8118
11 Turlings, T.C.J. et al. (1995) How
caterpillar-damaged plants protect themselves by attracting parasitic wasps, Proc. Natl. Acad. Sci. U. S. A. 92, 4169–4174
12 Boland, W., Hopke, J. and Piel, J. (1998)
Induction of plant volatile biosynthesis by jasmonates, in Natural Product Analysis,
Chromatography, Spectroscopy, Biological Testing (Schreier, P., Herderich, M.,
Humpf, H-U. and Schwab, W., eds), pp. 255–269, Viehweg-Verlag
13 Alborn, H.T. et al. (1997) An elicitor of plant
volatiles from beet armyworm oral secretions,
Science 276, 945–949
14 Mauricio, R. and Rausher, M.D. (1997)
Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense,
Evolution 51, 1435–1444
15 Kearsey, M.J. and Farquhar, A.G.L. (1998) QTL
analysis in plants; where are we now? Heredity 80, 137–142
16 Mitchell-Olds, T. and Pedersen, D. (1998) The
molecular basis of quantitative genetic variation in central and secondary metabolism in
Arabidopsis, Genetics 149, 739–747
17 Neuhaus, H.E. and Stitt, M. (1990) Control
analysis of photosynthate partitioning: Impact of reduced activity of ADP-glucose
pyrophosphorylase or plastid
phosphoglucomutase on the fluxes to starch and sucrose in Arabidopsis thaliana (L) Heynh.,
Planta 182, 445–454
18 Shirley, B.W. (1996) Flavonoid biosynthesis:
‘new’ functions for an ‘old’ pathway,
Trends Plant Sci. 1, 377–382
19 Clegg, M.T., Cummings, M.P. and Durbin, M.L.
(1997) The evolution of plant nuclear genes,
Proc. Natl. Acad. Sci. U. S. A. 94, 7776–7783 20 Tropf, S. et al. (1994) Evidence that stilbene
synthases have developed from chalcone synthases several times in the course of evolution, J. Mol. Evol. 38, 610–618
21 Hanson, M.A. et al. (1996) Evolution of
anthocyanin biosynthesis in maize kernels: the role of regulatory and enzymatic loci,
Genetics 143, 1395–1407
22 Kutchan, T. (1995) Alkaloid biosynthesis –
The basis for metabolic engineering of medicinal plants, Plant Cell 7, 1059–1070
23 Bohlmann, J., Meyer-Gauen, G. and Croteau, R.
(1998) Plant terpenoid synthases – molecular biology and phylogenetic analysis, Proc. Natl.
Acad. Sci. U. S. A. 95, 4126–4133
24 Langenheim, J. (1994) Higher plant terpenoids:
A phytocentric overview of their ecological roles,
J. Chem. Ecol. 20, 1223–1280
25 Broglie, K. et al. (1991) Transgenic plants with
enhanced resistance to the fungal pathogen
Rhizoctonia solani, Science 254, 1194–1197 26 Koiwa, H., Bressan, R.A. and Hasegawa, P.M.
(1997) Regulation of protease inhibitors and plant defense, Trends Plant Sci. 2, 379–384
27 Glazebrook, J. and Ausubel, F.M. (1994)
Isolation of phytoalexin-deficient mutants of
Arabidopsis thaliana and characterization of their
interactions with bacterial pathogens, Proc. Natl.
Acad. Sci. U. S. A. 91, 8955–8959
28 McConn, M. et al. (1997) Jasmonate is essential
for insect defense in Arabidopsis, Proc. Natl.
Acad. Sci. U. S. A. 94, 5473–5477
29 Feys, B.J.F. et al. (1994) Arabidopsis mutants
selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen,
Plant Cell 6, 751–759
30 Li, J. et al. (1992) Arabidopsis flavonoid mutants
are hypersensitive to UV-B irradiation, Plant Cell 5, 171–179
31 Purrington, C.B. and Bergelson, J. (1997) Fitness
consequences of genetically engineered herbicide and antibiotic resistance in Arabidopsis thaliana,
Genetics 145, 807–814
32 Stam, L.F. and Laurie, C.C. (1996) Molecular
dissection of a major gene effect on a quantitative trait: The level of alcohol dehydrogenase expression in Drosophila melanogaster, Genetics 144, 1559–1564
33 Rajaonarivony, J.I.M., Gershenzon, J. and
Croteau, R. (1992) Characterization and mechanism of (4S)-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha piperita), Arch.
Biochem. Biophys. 296, 49–57
34 Colby, S.M. et al. (1993) 4S-Limonene synthase
from the oil glands of spearmint (Mentha
spicata). cDNA isolation, characterization, and
bacterial expression of the catalytically active monoterpene cyclase, J. Biol. Chem. 268, 23106–23024
365
trends in plant science
update
September 1998, Vol. 3, No. 9
Book reviews in
Trends in Plant Science
Recently published books and CD-ROMs featured in Trends in Plant Science are assessed by experts in the field, providing an open peer-review service to potential purchasers. Authors are encouraged to give background information about the topic being discussed, and to assess the quality of the publication in this context. We also encourage our authors to express their personal viewpoint – as illustrated by the following examples:
‘I found myself continually making notes ...’
‘... conveys a brilliant, up-to-date vision.’
‘... an especially timely book ...’
‘... the book contains glaring scientific and editorial errors.’