www.elsevier.com / locate / bres
Research report
Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum
a,e d,e b,c a,b
Arvind K. Bansal
, Charles F. Mactutus
, Avindra Nath
, William Maragos
,
a a,e ,
*
Kurt F. Hauser , Rosemarie M. Booze
a
Department of Anatomy and Neurobiology, MN-224, University of Kentucky College of Medicine, 800 Rose Street, Lexington, KY 40536, USA b
Department of Neurology, University of Kentucky, Lexington, KY 40536, USA c
Department of Microbiology and Immunology, University of Kentucky, Lexington, KY 40536, USA d
Department of Pharmacy, University of Kentucky, Lexington, KY 40536, USA e
Graduate Center for Toxicology, University of Kentucky, Lexington, KY 40536, USA
Accepted 18 July 2000
Abstract
HIV-associated dementia complex is a serious disabling disease characterized by cognitive, behavioral and motor dysfunction. Basal ganglia involvement in HIV-1 infection may be responsible for some of the psychomotor symptoms associated with HIV dementia. The objectives of the present study were to determine: (1) whether gp120 and Tat produce striatal toxicity, and (2) whether gp120 and Tat show synergistic toxicity in the striatum. In these studies, the recombinant proteins gp120, Tat, or saline (0.9%) were stereotaxically injected in the striatum of adult male rats. The striatal sections were evaluated for area of tissue loss (Cresyl-violet stained sections) and the number of GFAP immunoreactive cells 7 days after the injections. Doses of gp120 250 ng /ml or higher and Tat 5mg /ml or higher produced a significant area of tissue loss and significantly increased the number of GFAP reactive cells. We found no toxicity in animals treated with immunoabsorbed gp120 or Tat. Combined gp120 (100 ng /ml)1Tat (1mg /ml) injections into the rat striatum significantly increased the area of tissue loss and altered morphology and increased number of GFAP reactive cells, as compared to controls. Thus, the present results suggest the involvement of gp120 and Tat in striatal toxicity and provide a model for further studies to fully characterize their role in HIV-1 toxicity and to develop therapeutic strategies for HIV-1 associated dementia complex. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Infectious diseases
Keywords: GFAP; Striatum; Astrocytes; In vivo; Dopamine
1. Introduction [12,37], however these beneficial effects may not be long lasting due to development of drug resistance. Therefore, HIV-associated dementia complex is a serious disabling to effectively treat or prevent HIV dementia, understanding disease developing in approximately 20% of the patient of its pathogenesis is essential. Towards that effort, population with advanced Human Immunodeficiency Virus recently, research has centered on the viral toxins, gp120 type 1 (HIV-1) infection [25]. HIV-associated dementia and Tat, as potential mediators of pathogenesis of HIV complex manifests in severely immuno-compromised pa- dementia complex [30].
tients and involves cognitive, behavioral and motor Gp120 is a coat glycoprotein and plays an important role dysfunction. With the development of highly active anti- in viral entry and in determining viral tropism [7]. Gp120 retroviral therapy (HAART) it is now recognized that may be shed during viral entry or may be released symptoms of HIV dementia are reversible in some patients extracellularly by infected cells [30]. Gp120 causes neuro-toxicity indirectly through activation of microglia and astrocytes [17]. Glial cells can secrete a variety of toxic *Corresponding author. Tel.: 11-859-323-6507; fax: 1
1-859-323-substances such as arachidonic acid metabolites, NMDA 5936.
E-mail address: [email protected] (R.M. Booze). agonists, platelet activating factor and various cytokines
such as TNF-alpha and IL-1, which are involved in First, we performed a dose–response study to determine neuronal injury [11,18]. In studies with the transgenic mice the concentrations of gp120 and Tat in the rat striatum that where gp120 is over-expressed the neuronal changes result in overt tissue loss and reactive astrocytosis. Second, resemble those in post mortem HIV infected human brains we assessed the specificity of gp120 and Tat toxicity by [39]. Thus, gp120 is a potential factor in the pathogenesis injecting solutions from which the proteins had been of HIV dementia; however the exact role of gp120 in the immunoabsorbed. Third, we determined whether gp120 pathogenesis remains unclear. and Tat together produced greater neurotoxicity than either Tat, a nonstructural protein of HIV, is also implicated in protein alone in the rat striatum. Thus, the goal of these the pathogenesis of HIV dementia. Tat is actively released studies was to establish an in vivo rodent model for from infected lymphoid and neuroglial cells [10]. Tat exploring the mechanisms of HIV-protein induced neuro-causes neurotoxicity by direct neuronal depolarization [6] toxicity within the basal ganglia. The importance of and by increasing levels of intracellular calcium and developing such a model of HIV protein neurotoxicity is cytokines. Tat, like gp120, may also cause indirect neuro- highlighted by the occurrence of psychomotor deficits and toxicity by activating excitatory amino acid receptors [20]. dopamine alterations in HIV-associated dementia complex. Even a brief exposure to Tat may stimulate a cascade of
events leading to neuronal death [28], suggesting the
possibility of an excitotoxic ‘hit and run’ mechanism in the 2. Materials and Methods CNS.
The basal ganglia are affected in HIV-1 infection with 2.1. Animals high viral load [43] and basal ganglia involvement may be
responsible for some of the psychomotor symptoms of Young (3–4 months old) Sprague–Dawley male rats HIV dementia. When patients with AIDS and symptoms of (approximate weight 300 g) were obtained from Harlan psychosis received dopamine-blocking drugs, they showed Laboratories (Indianapolis, IN). The animals were placed increased frequency and severity of extrapyramidal fea- in quarantine facilities for 1 week and then were moved to tures [26,15,35]. Reductions in dopamine, and its major the animal colony. Animals were maintained according to metabolite homovanillic acid, have been shown in caudate NIH guidelines in AAALAC accredited facilities. The nucleus specimens of AIDS patients [36] and in the CSF of animals were group housed (2 / cage) under controlled patients with HIV-associated dementia. The basal ganglia temperature (218C628C), relative humidity (50%610%) have among the highest levels of HIV RNA in AIDS and lighting (12l:12d, lights on at 0700 h) conditions. The patients [43]. In vitro treatment of rat midbrain cultures animals had ad libitum access to distilled water and a with gp120 produces reduction in uptake of dopamine and standard rat chow diet (Pro-Lab Rat, Mouse, hamster reduction in dopamine neuron process length, while not Chow No. 3000). No gross pathology was found in any affecting the total number of neuronal cells or dopamine animal used in this study.
containing cells [3]. In animals, treatment with gp120
reduces the ability of the neurons to transport dopamine [9] 2.2. HIV proteins and a recent study [45] suggested that Tat may inhibit
tyrosine hydroxylase expression in dopaminergic neurons. The recombinant gp120 was provided as a gift from Collectively, these in vivo and in vitro studies support the Chiron Corporation. Gp120 was produced in CHO cells hypothesis of gp120 and Tat involvement in basal ganglia from HIV-SF2 and was 100% pure. Recombinant Tat
damage by HIV. protein was produced as previously described [21]. We
Much work has been done studying the neurotoxic prepared solutions of gp120 in varying concentrations 400 effects of gp 120 and Tat in cell culture models; relatively ng /ml, 250 ng /ml, 100 ng /ml, 50 ng /ml, 10 ng /ml by few studies have been done with these proteins in animal adding sterile saline (0.9%). The lyophilized fraction of models focusing on neurotoxicity in dopaminergic brain Tat protein was used in varying concentrations of 50 regions. However, other studies of Tat injection into the mg /ml, 5mg /ml, 1 mg /ml.
brain parenchyma have found it to be a potent neurotoxin.
Injection of MVV tat peptide produced toxicity at 10 nmol 2.3. Surgical techniques and microinjections of peptide [13]. Infusion of basic domains of Tat into the
lateral ventricle or into the hippocampus or thalamus Standard stereotaxic surgery techniques were used for produced an inflammatory process [32]. A recent study protein injection. The instrument used for stereotaxic
according to a standard rat stereotaxic atlas [31]. The of 1 h and then directly transferred into a 1:500 dilution of coordinates used for striatum were: 0.3 mm anterior to affinity purified monoclonal mouse anti-Glial Fibrillary bregma, 2.5 mm lateral to bregma and 4.8 mm dorsal from Acidic Protein (anti-GFAP) (Boehringer Mannheim, Inc.) dura. The injection volume was 1ml injected over 1 min. and left overnight at room temperature. Following primary The injection began after a 1-min period letting the tissue antibody incubation, all sections were washed in PBS and come into original conformation, and was followed by a incubated for 1 h in mouse biotinylated secondary im-2-min period of needle removal so as to avoid needle munoglobulin (Vector Laboratories, Inc.). The avidin– reflux. After recovery from surgical anesthesia the animals biotin-complex technique (Elite ABC kit, Vector Labs, were returned to the vivarium. Burlingame, CA) was used to stain these tissue sections. Following a brief (approximately 5 min) reaction with 2.4. Experimental design 0.05% diaminobenzidine in 0.015% H O2 2 and enhanced with Nickel chloride (NiCl2, 8%) the sections were Animals were group housed and randomly distributed mounted on glass slides, air dried, dehydrated through a into gp120 (n512) and Tat (n512) treatment groups. series of alcohol / xylene solutions and coverslipped with Animals in the gp120 group were injected with a con- Vectamount (Vector Laboratories, Inc.)
centration of 400 ng /ml, 250 ng /ml, 100 ng /ml, 50 ng /ml Nissl solution was prepared from 20 mls of Cresyl-violet or 10 ng /ml. Animals in the Tat group were injected with a stock stain (0.1%) and 200 ml of buffer solution (0.1 M concentration of 50 mg /ml, 5 mg /ml or 1 mg /ml doses. sodium acetate / acetic acid, pH 3.9). Tissue sections to be Two animals were treated with 0.9 saline (vehicle). processed with Nissl were rinsed 33 in PBS. These To evaluate the specificity of our lesioning results, sections were then mounted on glass slides and rinsed in gp120 or Tat were immunoabsorbed with respective Nissl solution for 5–10 min. The slides were then rinsed in antisera (goat anti-gp120 antisera or rabbit anti-Tat anti- distilled water and dehydrated through a series of alcohol / sera) bound to protein A beads, and animals intracerebrally xylene steps and coverslipped with DPX (Aldrich Chemi-microinjected with the supernatants (n54; Imgp120 and cal Company, Inc.)
ImTat groups). Additionally, gp120 or Tat was treated with protein A beads bound to either normal goat serum or
2.6. Quantitative anatomical techniques normal rabbit serum, respectively, and intracerebrally
microinjected (n54; gp120 and Tat).
A computerized imaging system (Bioquant System, Additional animals (n53) were used in the gp1201Tat
R&M Biometrics, Nashville, TN) system was used to dosing experiment in which 100 ng gp12011mg Tat were
systematically identify GFAP-IR cells and determine the mixed (1 ml) and injected in the striatum. The control
area of striatal tissue loss [38]. GFAP-IR cells were groups for this latter experiment were injected with 100 ng
identified in those tissue sections showing the maximal gp120, 1mg Tat or saline.
area of tissue loss for each animal or, if no tissue loss, the injection tracks (1 section / animal). In vehicle-injected 2.5. Tissue processing
animals, the sections including the injection track were selected for quantitative analysis (1 section / animal). Four Seven days following injections the animals were
anes-different regions of the striatum were counted on the thetized with sodium pentobarbital (50 mg / kg
body-injected side and contralateral control side, excluding the weight). The animals were transcardially perfused with
injection track. A computerized grid was visualized over approximately 250 ml of 4% paraformaldehyde solution
the imaged striatum (approximately 60360 mm) with 100 and perfused brain was extracted. The brain was carefully
intersections and GFAP-IR cells were counted at 4003. blocked according to standardized anatomical landmarks
Every third square of the grid containing the striatum was and postfixed in 4% paraformaldehyde (pH 7.4) for 24 h.
counted. The cortical surface was notched on the non-injected side
The area of tissue loss was determined from Nissl prior to sectioning to orient the tissue when mounting on
stained sections by digitizing the tissue sections using the the glass slides. Tissue blocks were sectioned using a
Bioquant imaging system. Subsequently, computer-assisted vibrating microtome (Vibratome, Energy Beam Sciences).
tracing of the perimeter of the striatal tissue loss surround-The 40-mm thick sections were stored in cryoprotectant
ing the injection site was conducted to determine area solution until processed. Every fourth section was stained
measures. with Cresyl-violet Nissl-staining or with anti-Glial
Fibril-lary Acidic Protein (GFAP) for immunostaining
through-out the striatum. 2.7. Data analysis
The free-floating tissue sections were washed in
used (BMDP Statistical Software, Inc Los Angeles, CA) Similarly, a significant area of tissue loss was found in and planned comparisons used to determine specific treat- the animals treated with gp120 doses of 400 and 250 ment effects. Regression analysis was used to determine ng /ml. The area of tissue loss was graded in that a greater dose–response relationships [44]. In cases of completely area of loss was significantly greater in the 400 ng dose, as non-overlapping distributions (i.e., lesion size data), no compared to 250 ng dose (Fig. 1B). In contrast, there was statistics were calculated. An alpha level of P#0.05 was no tissue loss in the 100 ng /ml dose group animals. No the significance level for rejection of the null hypothesis. tissue loss was observed in striatum of the vehicle-injected
control group.
3. Results 3.2. Alterations in astrocyte morphology
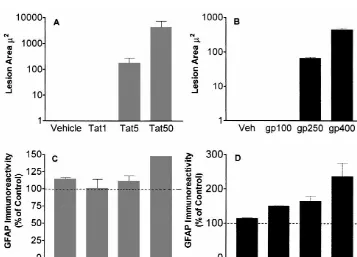
3.1. Striatal tissue loss following gp120 and Tat On GFAP immuno-stained sections the astrocytes in the
injection gp120 and Tat injected striatum demonstrated significant
alterations in morphology (Fig. 2). The astrocytes were At the highest intracerebral doses, both gp120 and Tat increased in size, and the number of arborizations were produced an area of tissue loss in the rat striatum (Fig. 1, also increased as compared to the control side. The Panels A and B) immediately surrounding the injection changes became less conspicuous distal from the lesion area. Overall, Tat produced a significantly greater area of area. In gp120 treatment group these alterations were tissue loss in the rat striatum, relative to the loss produced observed in 400 ng /ml and 250 ng /ml dose groups, by gp120. Significant striatal tissue loss occurred with whereas, no changes were observed in animals treated with intracerebral Tat doses of 50 and 5 mg. No significant 100 ng /ml dose. In Tat treatment group similar alterations tissue loss was observed in the 1mg dose group, relative to were seen in 50mg /ml dose treated animals, whereas, in the contralateral non-injected striatum or vehicle injected animals treated with 5 or 1 mg /ml doses no significant controls. The area of tissue loss was dose dependent, as alterations were seen as compared to the control side. more damage occurred with 50mg dose as compared to 5 Similarly, no significant changes in astrocyte morphology
mg dose (Fig. 1A). were seen in the striatum of vehicle injected animals.
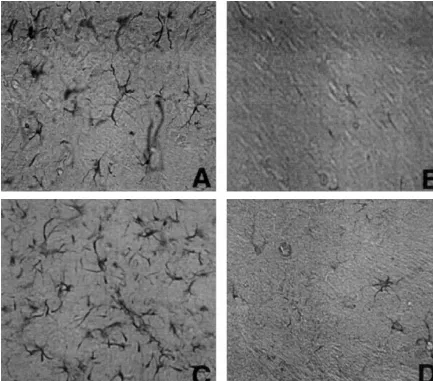
Fig. 2. GFAP immunoreactive cells following Tat and gp120 injection into the rat striatum. Tat injected striatum with increased size and number of GFAP immunoreactive astrocytes, Tat injected (A) and control (B). gp120 injected striatum with increased size and number of GFAP-immunoreactive astrocytes, gp120 injected (C) control (D). Magnification54003.
3.3. Increase in GFAP reactive cells following gp120 significant in 50mg /ml dose group, whereas 5mg /ml and
and Tat injections 1mg /ml groups were not significantly different from the
control or uninjected striatum (P#0.05). No significant There was a significant overall increase in GFAP-IR increase in GFAP-IR cells was seen in the striatum of positive cells in the gp120 and Tat injected striatum (Fig. vehicle-injected animals.
1, Panels C and D), relative to the contralateral
non-injected striatum [F(2,15)54.6, P#0.03]. The magnitude 3.4. Immunoabsorption of gp120 or Tat of this increase in GFAP-IR is significantly greater with
morphology as described above. Moreover, we have observed occasional microglia at the injection site of gp1201Tat (not shown). No tissue loss and no significant changes in astrocytes were seen in the control group receiving serum alone.
4. Discussion
In the present study, we examined the toxic effects of gp120 and Tat by microinjecting these proteins in the rat striatum. A dose–response study was performed to de-termine the concentrations of gp120 and Tat in the rat Fig. 3. No significant increase in GFAP-IR or tissue loss was seen striatum that resulted in tissue loss and astrocytosis. We following injection of solution from which gp120 or Tat had been
found microinjection of gp120 and Tat produced reactive immunoadsorbed [F(2,3)52.6, P,0.23], relative to injection of gp120
astrocytosis in a dose-dependent manner in the rat and Tat. A significant increase in the GFAP positive cells, and alterations
striatum. These effects were specifically mediated by in astrocyte morphology in the animals receiving gp120 (250 ng) and Tat
(5 mg) was noted as in the previous experiment. gp120 and Tat proteins, as determined by injecting solu-tions from which proteins had been immunoabsorbed. Moreover the independent and synergistic mechanisms of gp120 and Tat toxicity may be addressed. Gp120 and Tat 3.4.1. Toxicity of gp1201Tat together produced greater neurotoxicity than either protein The animals received sub-threshold toxic doses of gp120 alone in the rat striatum. Thus, the HIV-protein rodent or Tat (100 ng gp120, 1mg Tat) or combined gp1201Tat model may be useful for exploring the mechanisms of at the same concentrations (Fig. 4). Significant striatal HIV-protein induced neurotoxicity in the basal ganglia. tissue loss occurred in combined gp120 and Tat treated Reactive astrocytosis has been consistently shown to be animals. There was a significant increase in the number of an early change in the pathogenesis of HIV encephalitis GFAP positive cells with the combined gp1201Tat in- [5,41,42]. Astrocytosis may be a protective mechanism to jection relative to the Tat alone group [F(1,5)514.9, P# ward off the potential toxins from reaching the brain, or 0.012]. In contrast, the number of GFAP positive cells more significantly, this may compromise the blood brain attributable to gp120 alone was not significantly greater barrier function [4,8]. The evolving model of HIV-associ-than that observed for the serum-injected control group. ated dementia suggests the involvement of virotoxins (Tat, The significant increase in GFAP was associated with gp120, etc.) interacting with various cellular toxins (cyto-astrocytes that showed the characteristic alterations in kines, arachidonic acid metabolites, quinolinic acid, NO, NTOX etc.) [27,30]. It is thought that virotoxins activate glial cells to release various cellular toxins, which in turn adversely affect normal physiological functions of the neurons [30]. In this model of HIV-associated dementia astrocytes play an important role. Astrocytes may not only be infected by HIV [22] but uninfected cells may also be activated by gp120 and Tat to induce changes in intracellu-lar calcium [14] and release of cytokines (reviewed in [27]). Thus, activation of astrocytes may be an important early indicator of subsequent neuropathology.
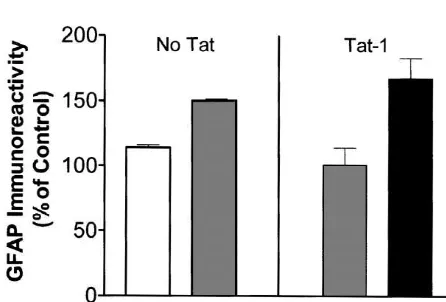
In previous in vitro studies we have shown that gp120 and Tat can cause synergistic neurotoxicity [29]. We now demonstrate that similar synergistic effects can occur in vivo. Moreover, the present study extends these observa-tions to show that gp120 and Tat may also synergize to produce astrocytosis in vivo. These observations are Fig. 4. The animals received either sub-threshold toxic doses of gp120 important as they suggest that much smaller concentrations and Tat (100 ng gp120, 1 mg Tat) or combined gp1201Tat at the same of these proteins may be necessary to induce neuro-concentrations. There was a significant increase in the number of GFAP pathological changes than previously thought, thereby positive cells with combined gp1201Tat injections [F(1,5)514.9, P5
supporting the role of these viral proteins as being an 0.012] as compared to the serum-injected control group. No tissue loss
important factor in the neuropathogenesis of HIV dementia and no significant changes in astrocytes were seen in the control group
In the current studies we assessed pathological changes tions related to lentiviral infection and to monitor the 7 days after a single injection of the viral proteins into the effects of neuroprotective therapy [33].
striatum. In the present studies we were also unable to Our alternative strategy was to use HIV protein toxins detect the presence of the injected viral proteins immuno- that have been implicated in the neuropathogenesis of HIV histochemically in the brain tissue after 7 days post- infection in a rodent model of striatal neurotoxicity. The injection (data not shown). Collectively, these data suggest knowledge base for the mechanisms of HIV protein that the continuous presence of the proteins is not neces- toxicity in vitro provides a substantial foundation for sary to produce pathological changes and as previously examining the proteins in vivo. Numerous in vitro studies suggested, a ‘hit and run’ phenomenon may be operative, have been done to elucidate the mechanisms of neuro-leading to a self-perpetuating cascade of degenerative toxicity of HIV proteins. For example, gp120 and Tat in events [28,29]. It is difficult to know what concentration of vitro produce excitatory amino acids, nitric oxide, arach-Tat in the brain is sufficient to produce pathogenesis. idonic acid, IL-1, and IL-6 (for review see [27]). However, However, we have recently been able to detect Tat mRNA in vitro techniques cannot provide the same information as and protein in tissue extracts of nine patients with HIV that obtained in the whole animal; for example, they encephalitis; Tat could also be detected in the brains of cannot provide data on behavioral changes reflecting rhesus macaques with encephalitis due to a chimeric strain damage to the basal ganglia. A rodent model to study the of HIV and simian immunodeficiency virus (SHIV) [16]. neurotoxicity of these proteins can help determine whether Concentrations of viral proteins detected in autopsy tissue these putative in vitro mechanisms are translational to are only representative of a single time point and thus may whole animals and ultimately to the pathology of HIV-not be reflective of their true pathogenic potential or associated dementia complex.
synergistic pathology in vivo. Direct microinjection of HIV-protein toxins that have In these studies we have developed a rodent model for been implicated in the neuropathogenesis of HIV infection exploring the mechanisms of HIV-protein induced neuro- provides a rodent model in which to examine mechanisms toxicity in the basal ganglia. Various studies have shown a of striatal neurotoxicity as well as toxic interactions with reduction in basal ganglia and posterior cortex and a more other risk factors. Since IV drug abusers are one of the generalized reduction in white matter on magnetic reso- major groups acquiring HIV infection [40], and most of nance imaging [1,2] in HIV-associated dementia. It has these drugs act on the dopaminergic systems, the study of been shown that people with HIV-associated dementia basal ganglia becomes even more compelling in animal have psychomotor slowing, also suggesting the in- models. Recent work suggests that Tat alone may regulate volvement of basal ganglia [24]. Dopamine alterations in key dopaminergic enzymes [45]. Therefore, in future HIV, together with high viral load in the basal ganglia [43], studies this model may be combined with existing models indicate that the basal ganglia may be a preferential target of intravenous drugs of abuse [23] to study the complex for the virus [19]. For these reasons, substantial energies interactions of drugs of abuse with HIV in producing the must be invested in developing in vivo and in vitro models symptomology of HIV-associated dementia complex. best suited to study the neurotoxicity of HIV infection,
especially with reference to the basal ganglia. However,
given that humans are the only host for HIV infection, Acknowledgements development of an entirely homologous animal model is
not possible. Therefore, careful consideration must be The authors gratefully acknowledge the assistance of C. given to the inherent problems and advantages of each Anderson and S. Chapman in the conduct of these studies. model system for exploring HIV-associated dementia. This work supported by NIH grants DA11337, DA09160, Primates can be infected with related retroviruses such NS39254, NS38184, NS / MH39253, DA06204, NS38428. as simian immunodeficiency virus (SIV) and a chimeric
strain of SIV and HIV. However, some important differ-ences still exist in the neurological outcome of these
References animals when compared to humans. For example, the onset
of symptoms is much more rapid in macaques and the
[1] E.H. Aylward, J.D. Henderer, J.C. McArthur, Reduced basal ganglia encephalitis much more fulminant than is typically seen in
volume in HIV-1 associated dementia: results from quantitative humans [34]. Furthermore, sample sizes in primates must
neuroimaging, Neurology 43 (1993) 2099–2104.
be limited due to the large amount of resources, expense [2] E.H. Aylward, P.D. Brettschneider, J.C. McArthur, Magnetic reso-and technical effort needed reso-and the scarcity of these nance imaging measurement of grey matter volume reductions in animals. The feline immunodeficiency (FIV) virus also HIV dementia, Am. J. Psychiatry 152 (1995) 987–994.
[3] B.A. Bennett, D.E. Rusyniak, C.K. Hollingsworth, HIV-1 gp120 causes encephalitis with neurodegeneration, although some
induced neurotoxicity to midbrain dopamine cultures, Brain Res. differences exist with respect to the neuropathology seen in
705 (1995) 168–176.
[5] R. Brack-Werner, Astrocytes: HIV cellular reservoirs and important slowed reaction time, and elevated cerebrospinal fluid quinolinic participants in neuropathogenesis, AIDS 13 (1999) 1–22. acid in a subgroup of HIV infected people, Neuropsychology 7 [6] J. Cheng, A. Nath, B. Knudsen, S. Hochman, J.D. Geiger, M. Ma, (1993) 149–157.
D.S.K. Magnuson, Neuronal excitatory properties of Human Im- [25] J.C. McArthur, O.A. Selnes, J.D. Glass, D.R. Hoover, H. Bacellar, munodeficiency Virus type 1 tat protein, Neuroscience 82 (1998) HIV Dementia. Incidence and Risk Factors, in: R.W. Price, S.W.
97–106. Perry (Eds.), HIV, AIDS and the Brain, Vol. 72, Raven, New York,
[7] F. Cocchi, A.L. DeVico, A. Garzigo-Demo, A. Cara, R.C. Gallo, P. 1994, pp. 251–272.
Lusso, The V3 domain of the HIV-1 gp120 envelope glycoprotein is [26] S.M. Mirsattari, C. Power, A. Nath, Parkinsonism with HIV critical for chemokine-mediated blockade of infection, Nat. Med. 2 infection, Mov. Disord. 13 (1998) 684–689.
(11) (1996) 1244–1247. [27] A. Nath, Pathobiology of Human Immunodeficiency Virus dementia, [8] L.M. Dallasta, L.A. Pisarov, J.E. Esplen, J.V. Werlet, A.V. Moses, Semin. Neurol 19 (2) (1999) 113–127.
J.A. Nelson, C.L. Achim, Blood–brain barrier tight junction disrup- [28] A. Nath, K. Conant, P. Chen, C. Scott, E.O. Major, Transient tion in Human Immunodeficiency virus-1 encephalitis, Am. J. Pathol exposure to HIV-1 tat protein results in cytokine production in 155 (6) (1999) 1915–1927. macrophages and astrocytes: a hit and run phenomenon, J. Biol. [9] E.B. Dreyer, S.A. Lipton, Toxic neuronal effects of the HIV coat Chem. 274 (24) (1999) 17098–17102.
protein gp120 may be mediated through macrophage arachidonic [29] A. Nath, N.J. Haughey, M. Jones, C. Anderson, J.E. Bell, J.D. acid, (Abstract) Society for Neuroscience Abstract, 20 (1994) 1049. Geiger, Synergistic neurotoxicity by human immunodeficiency virus [10] B. Ensoli, L. Buonaguro, G. Barilarri, B. Fiorelli, R. Gendelman, proteins Tat and gp120: protection by memantine, Ann. Neurol. 47
Release, uptake and effects of extracellular human immuno- (2000) 186–194.
deficiency virus type-1 tat protein on cell growth and viral replica- [30] A. Nath, J.D. Geiger, Neurobiological aspects of HIV infections: tion, J. Virol. 67 (1993) 277–287. neurotoxic mechanisms, Prog. Neurobiol. 54 (1998) 19–33. [11] L.G. Epstein, H.E. Gendelman, Human Immunodeficieny Virus [31] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates,
type-1 infection of the nervous system: pathogenic mechanisms, 4th Edition, Academic Press, London, 1998.
Ann. Neurol. 33 (1993) 429–436. [32] V. Philippon, C. Vellutini, D. Gambarelli, G. Harkiss, G. Arbuthnott, [12] C.G. Filippi, G. Sze, S.J. Farber, M. Shahmanesh, P.A. Selwyn, D. Metzger, R. Roubin, P. Filippi, The basic domain of the lentiviral Regression of HIV encephalopathy and basal ganglia signal intensity Tat protein is responsible for damages in mouse brain: Involvement abnormality at MR imaging in patients with AIDS after the initiation of cytokines, Virology 205 (1994) 519–529.
of protease inhibitor therapy, Radiology 206 (1998) 491–498. [33] M. Podell, K. Maruyama, M. Smith, K.A. Hayes, W.R. Buck, D.S. [13] M. Hayman, G. Arbuthnott, G. Harkiss, H. Brace, P. Filippi, V. Ruehlmann, L.E. Mathes, Frontal lobe neuronal injury correlates to Philippon, D. Thomson, R. Vigne, A. Wright, Neurotoxicity of altered function in FIV-infected cats, J. Acquired Immune Defic. peptide analogues of the transactivating protein Tat from maedi- Syndr 22 (1) (1999) 10–18.
visna virus and human immunodeficiency virus, Neuroscience 53 [34] R. Raghavan, E.B. Stephens, S.V. Koag, I. Adany, D.M. Pinson, Z. (1993) 1–6. Li, F. Jai, M. Sahni, C. Wang, K. Leung, L. Foresman, O. Narayan,
1 1
[14] C.P. Holden, N.J. Haughey, A. Nath, J.D. Geiger, Role of Na / H Neuropathogenesis of chimeric simian / human immunodeficiency exchangers, excitatory amino acid receptors and voltage-operated virus infection in pig-tailed and rhesus macaques, Brain Pathol. 7
21
Ca channels in human immunodeficiency virus type 1 gp120- (1997) 851–861. 21
mediated increases in intracellular Ca in human neurons and [35] G. Ramachandran, L. Glickman, J. Levenson, C. Rao, Incidence of astrocytes, Neuroscience 91 (1999) 1369–1378. extrapyramidal syndromes in AIDS patients and a comparison group [15] E. Hriso, T. Kuhn, J.C. Masdeu, M. Grundman, Extrapyramidal of medically ill patients, J. Neuropsychiatry Clin. Neurosci. 9 (4)
symptoms due to dopamine blocking agents in patients with AIDS (1997) 579–583.
encephalopathy, Am. J. Psychiatry 48 (11) (1991) 1558–1561. [36] A.M. Sardar, C. Czudek, G.P. Reynolds, Dopamine deficits in the [16] L. Hudson, J. Liu, A. Nath, M. Jones, R. Raghavan, O. Narayan, D. brain: the neurochemical basis of parkinsonian symptoms in aids,
Male, I. Everall, Detection of the human immunodeficiency virus Neuroreport 7 (4) (1996) 9–12.
regulatory protein tat in CNS tissues, J. Neurovirol. 6 (2000) [37] A.A. Skolnick, Protease inhibitors may reverse AIDS dementia,
145–155. JAMA (News) 279 (1998) 419.
[17] S.A. Lipton, HIV infected macrophages, gp120 and N-methyl-D- [38] M.L. Smith, R.M. Booze, Age related loss of cholinergic and aspartate receptor-mediated toxicity, Ann. Neurol. 33 (1993) 227– GABAergic neurons in the rat nucleus basalis, Neuroscience 67
228. (1995) 679–688.
[18] S.A. Lipton, AIDS related dementia and calcium homeostasis, Ann. [39] M.T. Stephanie, M. Elizer, M.R. Edward, F.R. Glenn, R.A. Carmela, NY Acad. Sci. 594 (1994) 189–196. M. Lennert, Central Nervous System damage produced by expres-[19] O.L. Lopez, G. Smith, C.C. Meltzer, J.T. Becker, Dopamine systems sion of HIV-1 coat protein gp120 in transgenic mice, Nature 367
in Human Immunodeficiency Virus-associated Dementia, Neuro- (1994) 188–193.
psychiatry Neuropsychol. Behav. Neurol 12 (3) (1999) 184–192. [40] UNAIDS, WHO, AIDS epidemic update: December 1999. [20] D.S. Magnuson, B.E. Knudsen, J.D. Geiger, R.M. Brownstone, A. [41] L. Vitkovic, A. da Cunha, Role for astrocytosis in HIV-1 associated
Nath, Human immunodeficiency virus type1 tat activates non-N- dementia, Curr. Top. Microbiol. Immunol. 202 (1995) 105–116. methyl-D-aspartate excitatory amino acid receptors and causes [42] S. Weis, H. Haug, H. Budka, Astroglial changes in the cerebral toxicity, Ann. Neurol. 37 (1995) 373–380. cortex of AIDS brains: a morphometric and immunohistochemcial [21] M. Ma, A. Nath, Molecular determinants for cellular uptake of Tat investigation, Neuropath. Appl. Neurobiol. 19 (1993) 329–335.
protein of human immunodeficiency virus type 1 in brain cells, J. [43] C.A. Wiley, V. Soontornniyomkij, L. Radhakrishnan, E. Masliah, J. Virol. 71 (1997) 2495–2499. Mellors, S.A. Hermann, P. Dailey, C.L. Achim, Distribution of brain [22] M. Ma, J.D. Geiger, A. Nath, Characterization of a novel binding HIV load in AIDS, Brain Pathol. 8 (1998) 277–284.
site for the human immunodeficiency virus type 1 envelope gp120 [44] B.J. Winer, Statistical Principles in Experimental Design, 2nd on human fetal astrocytes, J. Virol. 68 (1994) 6824–6828. Edition, McGraw Hill, New York, 1971.