GTP binding activity of membrane proteins in isolated barley
embryo is enhanced by abscisic acid
Karin Visser, Jan W. Kijne, Mei Wang *
Center for Phytotechnology,Leiden Uni6ersity/Netherlands Organization of Applied Research,Wassenaarseweg64,
2333AL Leiden,The Netherlands
Received 18 April 1999; received in revised form 1 July 1999; accepted 1 July 1999
Abstract
Pharmacological studies showed that an inhibitor (pertussis toxin) or activator (mastoparan) of the Ga-subunit of
hetero-trimeric GTP binding proteins can counteract the effect of the plant hormone abscisic acid (ABA) in the inhibition of barley embryo germination. The presence of several GTP binding proteins (ranging from 15 to 48 kDa) in membrane fractions of barley embryos was demonstrated by specific [a-32P]GTP labeling of proteins and by Western blotting using antibodies against highly
conserved G protein a-subunit or Ras protein. GTP binding was enhanced in protein fractions from ABA-treated embryos,
especially concerning poly-peptides of Mr 42, 29, 28, 27, and 25 kDa. Our data suggest that GTP-binding proteins are involved ABA-induced responses in barley embryo. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Abscisic acid; GTP binding proteins;Hordeum; Germination
www.elsevier.com/locate/plantsci
1. Introduction
The plant hormone abscisic acid (ABA) is in-volved in various processes such as stress tolerance and regulation of seed dormancy and germination [1 – 4]. ABA significantly inhibits barley grain ger-mination. Previously, we demonstrated that the inability of dormant barley grains to germinate under suitable imbibition conditions is correlated with a high endogenous level of ABA [4,5]. Em-bryos isolated from these dormant grains are able to germinate, and are much more responsive to externally applied ABA than are embryos isolated from non-dormant grains [5].
Several studies have been performed to deter-mine the site of ABA recognition at the cellular
level. Strong indications for a perception site at the plasma membrane have been found in both barley aleurone and guard cells [6,7]. However, intracellular perception sites may be present as well [8,9]. Both heterotrimeric guanyl-nucleotide-binding regulatory proteins (G proteins) and small G proteins (Ras-like proteins) play distinct roles in signal transduction processes in animal systems, and have recently been demonstrated to be impor-tant in plant signal transduction as well [10 – 14]. Since we are interested in the mechanism of action of ABA, we studied a possible role of G proteins
in ABA signal transduction. Previously, we
demonstrated the presence of GTP-binding
proteins in barley aleurone [15]. In this paper, we describe specific GTP binding to membrane proteins from barley embryos. Binding of GTP to some of these proteins was enhanced in ABA-treated embryos, consistent with a hypothesis that GTP-binding proteins are involved in ABA-in-duced responses in barley embryo.
* Corresponding author. Tel.: +31-71-5274914; fax: + 31-71-5274863.
E-mail address:[email protected] (M. Wang)
2. Materials and methods
2.1. Materials
Non-dormant barley grains (Hordeum distichum
L. cv Triumph) were obtained from Heineken Technical Services, The Netherlands (1989 har-vest). Dormant barley (H. distichum L. cv Tri-umph) was grown in a phytotron according to Schuurink et al. [16]. Grains were stored at
−20°C to preserve dormancy. [a-32P]GTP (3000
Ci/mmol) was purchased from Amersham.
2.2. Germination experiments
Embryos were isolated from dormant barley grains by using a scalpel. Ten embryos were incu-bated in 300 ml water in a 24-wells polystyrene plate, with or without ABA, pertussis toxin or mastoparan. Embryos were scored as being germi-nated if the roots were ]1 mm long. A germina-tion index (GI) was calculated [16], which gives a maximal value to embryos that germinated first and less value to those that germinated later:
GI=(3×n1+2×n2+1×n3)
3×total embryos
in which ni represents the number of germinated
embryos at n1=24 h, n2=32 h and n3=48 h,
respectively. The maximal value of GI is 1.0 and the minimal value is 0.0.
2.3. Isolation of membrane protein fractions
Embryos were isolated from non-dormant bar-ley grains by using a scalpel. Embryos (fresh weight 3 mg) were incubated for 24 h in 10 ml water in a 9 cm Petri dish. Membrane protein fractions were isolated following the method de-scribed by Novikova et al. [13]. Briefly, the em-bryos were ground in liquid nitrogen and the powder was re-suspended in buffer A (50 mM
HEPES, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT,
1 mM PMSF, 250 mM sucrose, pH 7.6). All following steps were carried out at 4°C. After centrifugation at 10 000 g, the supernatant was centrifuged at 100 000 g for 1 h to yield a mal pellet and a cytosolic fraction. The microso-mal pellet was re-suspended in buffer B (25 mM
HEPES, 5 mM MgCl2, 1 mM EDTA, 0.5 mM
DTT, pH 7.6) containing 100 mM KCl, then
shaken for 30 min at 4°C and centrifuged at 100 000g for 1 h. The resulting supernatant was called the S1 fraction. The pellet fraction was resuspended in buffer B containing 750 mM KCl, shaken and centrifuged as described before. The resulting supernatant, called the S2 fraction, was collected. The pellet was re-suspended in buffer B containing 0.1% Triton X-100, and centrifuged as described before. The resulting final supernatant fraction was called S3. The supernatant fractions S1, S2 and S3 were dialyzed overnight against
buffer C (25 mM HEPES, 5 mM MgCl2, 5 mM
KCl, pH 7.6) and stored at −20°C until further use for GTP affinity labeling.
2.4. Affinity labeling with [a-32P]GTP
Labeling of proteins with [a-32P]GTP was
per-formed according to Lo¨w et al. [17] and Novikova et al. [13]. Reaction mixtures of 100 ml buffer C containing 25 – 50 mg protein and 5 mCi of [a
-32P]GTP were incubated for 10 min at 37°C. Then,
NaIO4 was added to a final concentration of 4
mM and oxidation was allowed to proceed for 1
min at 37°C. Subsequently, NaCNBH3was added
to a final concentration of 80 mM, followed by further incubation for 1 min at 37°C. Then, NaBH4was added to a final concentration of 100
mM and incubation was continued for 1.5 h at 0°C. The specificity of the labeling was tested by addition of a 100-fold excess of unlabeled GTP, CTP, GDP or ATP to the reaction mixture. Protein labeling was judged from SDS-PAGE fol-lowed by autoradiography.
2.5. Western analysis
Protein concentrations in the fractions were de-termined by using the protein assay with BCA (Pierce). Protein fractions of 20 – 50 mg were sepa-rated by SDS-PAGE (12.5%) and electrophoreti-cally transferred to a nitrocellulose membrane. These membranes were blocked in phosphate-buffered saline containing 0.05% Tween-20 and 1% BSA for 1 h at room temperature, and subse-quently incubated overnight at 4°C with 1:500 diluted rabbit antiserum raised against Ga
-com-mon peptide (Dupont, NEN) or with an anti-Ras
monoclonal antibody (H-Ras 259, Santa Cruz).
or rat IgG according to the ProtoBlot Western blot AP system technical manual (Promega).
3. Results
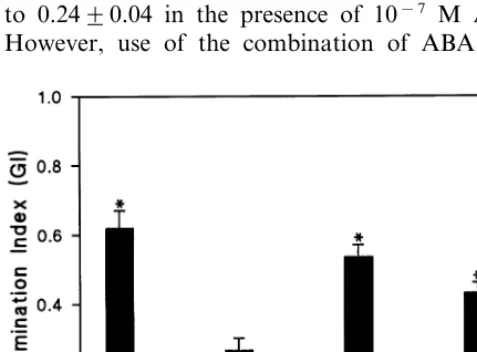
3.1. Effect of G-protein (ant)agonists on germination of embryos
Intact dormant barley grains fail to germinate when incubated on wet filter paper under suitable laboratory conditions [5]. However, embryos iso-lated from these grains start to germinate after 24 h. Albeit able to germinate, these embryos fail to
do so in 10−5 M ABA. Incubation in 10−7 M
ABA resulted in about 50% inhibition of germina-tion (Fig. 1). To test whether heterotrimeric G-proteins might be involved in the response to ABA, the Gasubunit inhibitor pertussis toxin and
activator mastoparan were used. Embryos isolated from dormant grains were incubated in water with ABA, pertussis toxin, mastoparan or combina-tions of these compounds. When isolated embryos germinated in water, the germination index (GI) was 0.6290.05. The germination index decreased to 0.2490.04 in the presence of 10−7 M ABA.
However, use of the combination of ABA with
pertussis toxin (50 ng/mL) or mastoparan (10mM) (concentrations normally used in comparable re-search) increased the germination indices as com-pared with ABA treatment (Fig. 1). The embryos germinated with a GI similar to that of the water control when only pertussis toxin (50 ng/mL) or
mastoparan (10 mM) were added (data not
shown). These data suggest that heterotrimeric G-proteins are involved in the inhibitory effect of ABA on germination of isolated barley embryos.
3.2. Detection of GTP-binding proteins in barley embryo
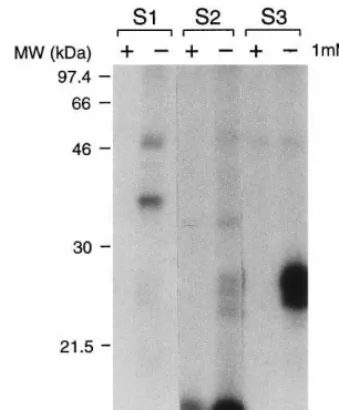
As a follow-up of the pharmacological ap-proach, we tested the working hypothesis that ABA promotes GTP binding by putative G-proteins. GTP binding studies were carried out with protein extracts from barley embryos using the method described by Novikova et al. [13] and Lo¨w et al. [17]. First, the presence of GTP-binding proteins was tested for different membrane frac-tions of isolated barley embryos. Isolated embryos were allowed to germinate in water for 24 h, protein extracts were prepared, and three different membrane protein fractions (S1, S2 and S3) were obtained. [a-32P]GTP was used to specifically label
proteins in the extracts. Three major distinct poly-peptides with molecular weights of 48, 42 and 39 kDa, respectively (Fig. 2) were labelled with ra-dioactive GTP in the S1 fraction. In the S2 frac-tion, one polypeptide with a molecular weight of about 36 kDa and three minor poly-peptides with molecular weights of about 28, 27 and 25 kDa were labeled with radioactive GTP (Fig. 2). In this fraction, also a prominent polypeptide with a molecular weight of 15 kDa was labeled with [a-32P]GTP, but binding of [a-32P]GTP to this
polypeptide could not be completely prevented by addition of GTP (Fig. 2). In fraction S3, two poly-peptides with molecular weights of about 26 to 25 kDa were labeled with radioactive GTP (Fig. 2). The addition of a 100-fold excess of non-ra-dioactive GTP was able to prevent specific [a
-32P]GTP binding activity (Fig. 2).
3.3. ABA enhances the binding of GTP to specific proteins
The effect of the plant hormone ABA on the binding of GTP to proteins from non-dormant
Fig. 1. Effect of pertussis toxin, mastoparan and ABA on germination of isolated dormant barley embryos. Ten em-bryos were incubated in water, ABA (10−6 M), pertussis
toxin (PT) (50 ng/ml), mastoparan (MP) (1mM) or
Fig. 2. Affinity labeling of GTP-binding membrane protein fractions isolated from non-dormant barley embryos. S1, S2 and S3 membrane protein fractions were incubated with 5mCi
[a-32P]GTP in the presence (+) or absence (−) of unlabeled
GTP (final concentration 1 mM). Proteins (approximately 10 – 20mg/lane) were separated by SDS-PAGE and visualized
by autoradiography. One typical experiment of four is pre-sented.
bryos were used. Embryos were isolated from non-dormant grains and incubated for 24 h in
water or in 10−4 M ABA. Incubation in water
resulted in 100% germination whereas of the ABA-treated embryos approximately 50% germinated. Subsequently, membrane protein fractions were isolated from these embryos as described in Sec-tion 2, and used in the GTP binding assay. Incu-bation in ABA enhanced the labeling with
[a-32P]GTP to poly-peptides with molecular
weights of 48, 42 and 39 kDa (Fig. 3) in fraction S1. A strong stimulation of labeling with [a
-32P]GTP to three proteins with molecular weights
of about 28, 27 and 25 kDa was detected in fraction S2 from ABA-treated embryos (Fig. 3). Moreover, this fraction contained an additional [a-32P]GTP labeled polypeptide with a molecular
weight of about 29 kDa (Fig. 3). ABA treatment did not result in stimulation of [a-32P]GTP binding
by poly-peptides in fraction S3 (Fig. 3). The ob-served increase of GTP binding was not due to unequal loading of proteins, as judged from Coomassie Brilliant Blue staining of the gels (data not shown).
Prominent stimulation of GTP binding to a polypeptide with a molecular weight of 29 kDa was observed in the S2 fraction. The specificity of the labeling was tested for this fraction by use of unlabeled GTP, GDP, CTP or ATP. The binding of [a-32P]GTP to the proteins with molecular
weights of about 28 – 25 kDa could be prevented with GTP and GDP, but not with CTP or ATP (Fig. 4). This indicates a specific binding capacity of these proteins for GTP and GDP. The binding of [a-32P]GTP to the smaller proteins in this
frac-tion was partially inhibited in the presence of GTP and GDP.
3.4. Western analysis of GTP binding proteins from barley embryos
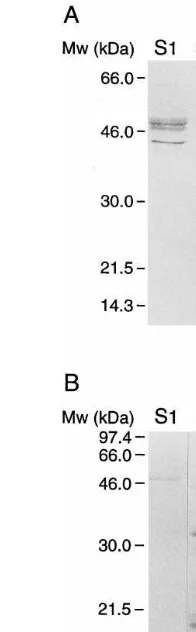
To test whether the GTP-binding proteins de-tected with the GTP-binding assay were proteins of the G-protein family, Western analysis was performed. Similar protein extracts as were used for the GTP-binding assay (S1, S2 and S3 frac-tions) were separated by SDS-PAGE, transferred to nitrocellulose, and probed with an antibody raised against Ga-common peptide (Fig. 5A).
Sev-eral poly-peptides were detected by this antibody in the S1 fraction, and these poly-peptides had
Fig. 3. Effect of ABA treatment on binding of GTP to barley embryos. Barley embryos isolated from non-dormant grains were incubated overnight in water with or without 10−4 M
ABA. Membrane protein extracts (see materials and methods) were prepared and affinity labeling with [a-32P] GTP was
performed on S1, S2 and S3 fractions. Proteins were sepa-rated and radioactive poly-peptides were detected by autora-diography. One of three typical experiments is shown.
em-molecular weights of about 48 and 42 kDa, similar to those of two of the GTP-labeled poly-peptides. The Ga antibody did not label proteins in the S2
and S3 fractions. This correlation suggests that GTP binding to two heterotrimeric G-proteins like proteins was stimulated in the S1 fraction from ABA treated embryos.
Another antibody, anti H-Ras, recognized sev-eral poly-peptides with molecular weights of about 17, 22 – 26, 29 and 33 kDa in the S2 and S3 fractions (Fig. 5B). Only one polypeptide of 48 kDa was recognized in the S1 fraction. The poly-peptide with a molecular weight of about 29 kDa corresponded with the newly labeled protein in fraction S2 from ABA-treated embryos (Fig. 4.). This correlation suggests it is possible that GTP-binding to small GTP-GTP-binding proteins was en-hanced in ABA treated embryos.
4. Discussion
Previously, we detected GTP-binding proteins with molecular weights ranging from 16 to 24 kDa in barley aleurone, as shown by a GTP binding assay on Western blots [15]. However, we were not able to show an effect of ABA on GTP binding by poly-peptides in barley aleurone protoplasts [15].
Fig. 5. Western analysis of different membrane protein frac-tions. Protein fractions S1, S2 and S3 (50mg) were separated
by SDS-PAGE and transferred electrophoretically to nitrocel-lulose. A: Detection of proteins with a polyclonal antibody against Ga-subunit peptide. B: Detection with a monoclonal
antibody against H-Ras(Santa Cruz) (dilutions 1:250). Blots were developed with rabbit (panel A) or goat-anti-rat (panel B) antibodies coupled to alkaline phosphatase
Fig. 4. Specificity of GTP binding to proteins in different membrane protein fractions from isolated non-dormant bar-ley embryo. S2 protein fractions were affinity-labeled with [a-32P]GTP in the absence or presence of 1 mM unlabeled
GTP, GDP, ATP, CTP or 10−4M ABA (lane A). Proteins
were separated by SDS-PAGE and labeling patterns were visualized by autoradiography. One of three typical experi-ments is presented.
Germination of non-dormant embryos is not completely inhibited by ABA (10−4M) but merely
delayed. Incubation of isolated embryos in ABA results in a different germination behavior, in that at a certain time point, the germination stage of embryos in water is more progressed than that of embryos incubated in ABA. However, the ob-served effect of ABA on GTP binding was likely not due to a difference in germination stage. Em-bryos germinated in water for 4 h compared with embryos germinated in water for 24 h showed no difference in GTP-binding pattern of the proteins (data not shown).
The Ga-subunit inhibitor and activator,
pertus-sis toxin (PT) and mastoparan (MP), respectively, showed a counteracting effect to ABA with regard to the inhibition of embryo germination (Fig. 1). Both PT (inhibitor) and MP (activator) showed a stimulatory effect on embryo germination. This result is surprising since it is expected for an inhibitor and an activator to show an opposite effect. However, the finding might be explained if multiple pathways exist in ABA mediated inhibi-tion of germinainhibi-tion. This might be the case when
PT acts on Ga-inhibition by ABA and MP
di-rectly acts on Ga-activation, addition of these
antagonist can result in a stimulation of
germination.
In Arabidopsis, it has been reported that a cDNA coding for a 42 kDa protein shows very conserved homology to animal Ga-subunit [18].
Western analysis demonstrated the presence of polypeptides with a molecular weight of 42 and 43 kDa that cross-reacted with an antibody against Ga-common peptide (Fig. 5). GTP binding
activ-ity was detected to a protein with a molecular weight of about 42 kDa in the S1 fraction, which was stimulated by ABA treatment (Figs. 2 and 3). Therefore, it is possible that ABA enhanced GTP binding to a 42 kDa protein from the S1 fraction is involved in ABA action.
The presence of small GTP binding proteins with a molecular weight of 21 – 27 kDa was demonstrated. Stimulation of GTP binding to poly-peptides of 25, 27 and 28 kDa was detected for protein fractions from ABA-treated embryos. In addition, a 29 kDa polypeptide bound GTP only in the fraction from ABA-treated embryos (Figs. 2 and 3). These small G proteins could represent GTPases or Ras-like proteins. It was recently demonstrated in aleurone protoplasts,
that ABA-induced gene expression is involved in the activation of a MAP kinase [19]. Since it has been demonstrated that Ras protein is involved in MAP kinase kinase activation in animal systems [10] and that Ras or Ras-like proteins are also present in plants [20], it is possible that ABA-stim-ulated small G-protein activation might be in-volved in ABA-induced gene expression.
Combining our experimental data, we hypothe-size that the inhibitory effect of ABA on germina-tion of barley embryos involves Ga or Ga-like
proteins or small G-proteins whereas certain ABA-induced gene expression [19] may involve small G-proteins. Thus, it is possible that these two events use different or at least partial different signal transduction pathways.
References
[1] M. Black, Involvement of ABA in the physiology of developing and mature seeds, in: W.J. Davies, H.G. Jones (Eds.), Abscisic Acid: Physiology and Biochem-istry, BIOS, Scientific Publishers, Oxford, 1991, pp. 99 – 124.
[2] H.W.M. Hilhorst, C.M. Karssen, Seed dormancy and germination: the role of abscisic acid and gibberellins and the importance of hormone mutants, Plant Growth Regulation 11 (1992) 225 – 238.
[3] J. Giraudat, F. Parcy, N. Bertauche, F. Gosti, J. Leung, P.C. Morris, M. Bouvier-Durand, N. Vartanian, Current advances in abscisic acid action and signalling, Plant Mol. Biol. 26 (1994) 1557 – 1577.
[4] M. Wang, S. Heimovaara-Dijkstra, B. Van Duijn, Mod-ulation of germination of embryos isolated from dor-mant and non-dordor-mant barley grains by manipulation of endogenous abscisic acid, Planta 195 (1995) 586 – 592. [5] J.M.M. Van Beckum, K.R. Libbinga, M. Wang, Abscisic
acid and gibberellic acid-regulated responses of embryos and aleurone layers isolated from dormant and nondor-mant barley grains, Physiol. Plant. 89 (1993) 483 – 489. [6] S. Gilroy, R.L. Jones, Perception of gibberellin and
abscisic acid at the external face of the plasma membrane of barley (Hordeum6ulgareL.), Plant Physiol. 104 (1994)
1185 – 1192.
[7] B.E. Anderson, J.M. Ward, J.I. Schroeder, Evidence for an extracellular reception site for abscisic acid in Com-melinaguard cells, Plant Physiol. 104 (1994) 1177 – 1183. [8] A.C. Allan, M.D. Fricker, J.L. Ward, M.H. Beale, A.J. Trewavas, Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells, Plant Cell 6 (1994) 1319 – 1328.
[9] S. Gilroy, Signal transduction in barley aleurone proto-plasts is calcium dependent and independent, Plant Cell 8 (1996) 2193 – 2209.
[11] P.A. Millner, B.E. Causier, G-protein coupled receptors in plant cells, J. Exp. Botany 47 (1996) 983 – 992.
[12] H.D. Jones, S.J. Smith, R. Desikan, S. Plakidou-Dymock, A. Lovegrove, R. Hooley, Heterotrimeric G-proteins are implicated in gibberellin induction of a-amylase gene
expression in wild oat aleurone, Plant Cell 10 (1998) 245 – 253.
[13] G. Novikova, I. Moshkov, A.R. Smith, M.A. Hall, The effect of ethylene on GTP binding in extracts from pea epicotyls, Planta 201 (1997) 1 – 8.
[14] S.M. Assman, Guard cell G proteins, Trends Plant Sci. 1 (1996) 73 – 74.
[15] M. Wang, N.J.A. Sedee, F. Heidekamp, B.E. Snaar-Jagal-ska, Detection of GTP-binding proteins in barley aleurone protoplasts, FEBS Lett. 329 (1993) 245 – 248.
[16] R.C. Schuurink, J.M.M. Van Beckum, F. Heidekamp,
Modulation of grain dormancy by variation of plant growth conditions, Hereditas 117 (1992) 137 – 143. [17] A. Lo¨w, H.G. Faulhammer, M. Sprinzl, Affinity labelling
of GTP-binding proteins in cellular extracts, FEBS Lett. 303 (1992) 64 – 68.
[18] H. Ma, M.F. Yanofsky, E.M. Meyerowitz, Molecular cloning and characterization of GPA1, a G protein alpha subunit gene fromArabidopsis thaliana, Proc Natl Acad Sci. USA 87 (1990) 3821 – 3825.
[19] M.L.W. Knetsch, M. Wang, B.E. Snaar-Jagalska, S. Heimovaara-Dijkstra, Abscisic acid induces mitogen-acti-vated protein kinase activation in barley aleurone proto-plasts, Plant Cell 8 (1996) 1061 – 1067.
[20] T. Haizel, T. Merkle, F. Turck, F. Nagy, Characterization of membrane bound small GTP-binding proteins from Nicotiana tabacum, Plant Physiol. 108 (1995) 59 – 67.