Cytokinesis in plant cells is accomplished when a membranous cell plate is guided to a pre-established division site. The orientation of the new wall establishes the starting position of a cell in a growing tissue, but the impact of this position on future development varies. Recently, proteins have been identified that participate in forming, stabilizing and guiding the cell plate to the correct division site. Mutations that affect cytokinesis with varying impacts on plant development are providing information about the mechanics of cytokinesis and also about how the division site is selected.
Addresses
Department of Botany, PO Box 3165, University of Wyoming, Laramie, Wyoming 82071-3165, USA; e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:58–66
1369-5266/00/$ — see front matter © 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations dcd discordia
KCBP kinesin-like calmodulin binding protein
KatAp kinesin from Arabidopsis thaliana A peptide
MAPK mitogen-activated protein kinase
MMK3 Medicagomitogen-activated protein kinase 3
MT microtubule
NPK1 Nicotianaprotein kinase 1
PP phragmoplast
PPB preprophase band
TKRP125 tobacco kinesin-related polypeptide of 125 kDa
wty1 warty1
Introduction
Plant cell division serves a dual function of increasing cell number and also positioning new cross walls during cytoki-nesis. Once the new wall is formed, cells acquire a final shape by differential cell expansion. Several important decisions are thus made during division and expansion, including when and how often division should occur, where a new cell wall should be located, and in what direc-tion to expand. The outcome of any one of these decisions could be critical to the successful development of an organ because new daughter cells are joined from inception by cell walls. In reality, however, the position of new walls has a variable influence on the shape of an organ or function of a tissue. This is because the spatial regulation of cytokine-sis, which determines where new walls will be positioned, affects development to different degrees [1]. Regardless of the impact of division pattern on development, successful cytokinesis is needed to maintain the integrity of the plant body. Understanding how and when cytokinesis occurs will help to evaluate the effect of different stages of develop-ment on its spatial regulation.
Cytokinesis is accomplished by progressive deposition of membranes and associated wall synthesizing compounds into a cell plate, which is first located centrally in the
equatorial zone between recently formed daughter nuclei [2–5]. The growing cell plate then spreads out radially, as an expanding interlacing membranous network, until it joins with the parental walls at the periphery. A cytoskeletal structure called a phragmoplast (PP) not only guides vesicles to the equatorial zone but also directs the growing cell plate toward a specific site at or near the membrane of the parental cell wall — the cortical division site. This ill-defined site is first evident prior to mitosis when the preprophase band (PPB) appears transiently as a continuous band of cytoskeletal elements in the cell cortex. Remarkably, the position of the PPB forecasts the future site of attachment between the cell plate and the parental cell walls. The integrated behavior of these cytoskeletal structures thus accomplishes the feat of depositing a new wall at a specific position. What is the mechanism of cell plate formation? What determines where this new wall will form? How does cell position affect future development? This review presents recent progress in answering these questions and also evaluates potential versatility in the process, considering that some divisions have more impact on future development than others. Recent advances in understanding cell cycle reg-ulation [6], mechanisms of mitosis [7] or cell expansion [8,9 (DJ Cosgrove, pp 73–78)] are relevant topics, but are not covered here. Excellent diagrams showing the detailed structure of the growing cell plate and PP are available elsewhere [2,4,5].
Forming the cell plate
Completion of cytokinesis requires the interaction of fus-ing secretory vesicles, dynamic microtubules (MTs), actin and numerous associated proteins (Figure 1), as confirmed by electron and immunofluorescence microscopy and by experimental manipulation of cytokinesis. Disruption of the Golgi complex with the inhibitor brefeldin A, for example, prevents completion of the cell plate, suggesting that new vesicles are Golgi-derived [10]. Vesicles will also not accumulate in the equatorial zone of cells treated with inhibitors that depolymerize MTs [11], as a result of either transport inhibition or altered organization of the Golgi complex [12]. On the other hand, taxol, which hyperstabi-lizes MTs by preventing their depolymerization, increases the number of vesicles in the equatorial zone initially, although cytokinesis is eventually arrested [13]. These results demonstrate that MTs must be present but not nec-essarily dynamic for successful vesicle transport, supporting the idea that vesicles migrate along MTs with the aid of motor proteins [14].
Vesicle transport
The exact mechanism of vesicle transport is still an open question. The bipolar MTs in the PP are arranged with their plus ends overlapping in the equatorial zone and
Division decisions and the spatial regulation of cytokinesis
minus ends directed toward the daughter nuclei (Figure 1), suggesting vesicles might be transported using a plus-end directed motor protein [5]. A candidate kinesin-related protein isolated from tobacco PPs (TKRP125) has the expected domains for motor activity [15]. The protein localizes, however, along the entire length of MTs in the PP, rather than in the punctate pat-terns expected for vesicle association. TKRP125 could still interact with other as yet unidentified proteins asso-ciated with vesicles. In permeablized cells, however, antibodies against TKRP125 actually inhibit plus-end MT translocation [15]. Together, these results suggest that TKRP125 might contribute to maintaining the struc-ture of the MTs in the PP, rather than transporting vesicles to the cell plate.
Minus-end directed kinesin-like proteins have been found associated with the PP, including kinesin-like calmodulin binding protein (KCBP) and a kinesin from Arabidopsis thalianaA peptide (KatAp). KCBP mediates MT translo-cation in vitro[16] and its motor domain binds MTs [17•].
KCBP localizes to the MTs in the PP and also to the nuclear membrane early in telophase and within the equa-torial zone [18•]. KatAp is initially present in the spindle
mid-zone before the PP is organized and then in associa-tion with the PP itself [19]. The localizaassocia-tion patterns for both proteins are consistent with a function in organizing the MTs in the PP, rather than in directing vesicle transport [18•,19], particularly as similar kinesins in other systems
help to assemble and organize the mitotic spindle [20]. The translocation of MTs toward the minus end could pro-pel vesicles toward the plus ends. Most evidence, however,
points to an organizational function for these proteins, per-haps mediated by these minus-end directed motors.
F-actin could be a candidate for vesicle motility because it co-localizes with MTs in the PP, and is oriented as expected for a motility system [21]. Myosin may also be present in the PP [22]. Although disruption of actin filaments with cytocha-lasin does not affect vesicle accumulation, it does disorient the cell plate [11]. F-actin could facilitate vesicle transport through stabilizing the MTs, as discussed below but whether it plays a direct role in vesicle transport is not clear.
Organizing the phragmoplast
MT translocation might help to establish and then main-tain the bipolar MT array of the PP [23], possibly mediated by the kinesin-related proteins discussed above. Differential MT depolymerization is also important in PP function [10]. Centrifugal growth of the cell plate is pre-vented when MTs are stabilized, mirroring an effect of preventing depolymerization on spindle function [7]. These studies point to a required balance between poly-merization at the leading edge and depolypoly-merization at the trailing edge of the PP. It is likely that similar organiza-tional mechanisms are found for the PP as found for other MT structures, such as the mitotic spindle.
Vesicle accretion and fusion in the cell plate
Newly arriving vesicles coalesce into a membranous network as the cell plate extends out radially [4]. Clathrin-coated vesi-cles are observed in conjunction with the growing tubulo-vesicular network of the cell plate, providing evi-dence for fusion and membrane recycling as part of cell plate Figure 1
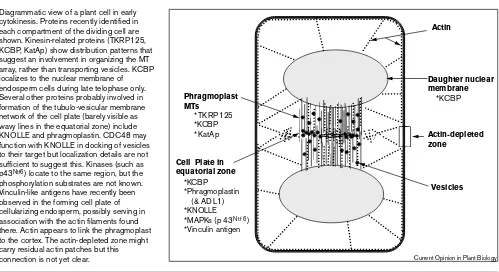
Diagrammatic view of a plant cell in early cytokinesis. Proteins recently identified in each compartment of the dividing cell are shown. Kinesin-related proteins (TKRP125, KCBP, KatAp) show distribution patterns that suggest an involvement in organizing the MT array, rather than transporting vesicles. KCBP localizes to the nuclear membrane of endosperm cells during late telophase only. Several other proteins probably involved in formation of the tubulo-vesicular membrane network of the cell plate (barely visible as wavy lines in the equatorial zone) include KNOLLE and phragmoplastin. CDC48 may function with KNOLLE in docking of vesicles to their target but localization details are not sufficient to suggest this. Kinases (such as p43Ntf6) locate to the same region, but the
phosphorylation substrates are not known. Vinculin-like antigens have recently been observed in the forming cell plate of cellularizing endosperm, possibly serving in association with the actin filaments found there. Actin appears to link the phragmoplast to the cortex. The actin-depleted zone might carry residual actin patches but this connection is not yet clear.
Actin-depleted zone
Actin
Daughter nuclear membrane *KCBP Phragmoplast
MTs
*TKRP125 *KCBP *KatAp
Cell Plate in equatorial zone
*KCBP *Phragmoplastin (& ADL1) *KNOLLE *MAPKs (p43Ntf6)
*Vinculin antigen
Vesicles
formation [4]. A protein that probably facilitates this process is phragmoplastin, a dynamin-related protein originally iso-lated from soybean [24] and also from Arabidopsis (called Arabidopsisdynamin-like protein 1 [ADL1]) [25]. In animal systems, dynamin is a GTPase that functions in endocytosis, particularly in membrane separation [26]. Both phragmoplas-tin and ADL1 localize to the growing cell plate [24,27]. Phragmoplastin is not dependent on MT organization, as it remains at the cell plate margins after the PP has dissociated near the end of cytokinesis. Taxol-stabilization of MTs does not eliminate phragmoplastin from its earliest position in the equatorial zone but does prevent its redistribution to the leading edge [24]. In vivoobservations using green-fluores-cent protein (GFP)-fusion proteins show that phragmoplastin associates with sites of high membrane activ-ity [28], consistent with a dynamin-like role for the protein.
Many essential protein functions are regulated by phos-phorylation, including dynamin assembly and kinesin activity [29,30]. Recently, mitogen-activated protein kinas-es (MAPKs) were found in the cell plate. MAPKs are cell-cycle regulated proteins involved in organizing the cytoskeleton and are also abundant in diverse signaling pathways [31,32]. Two proteins similar to MAPKs, (p43Ntf6
from tobacco and Medicago mitogen-activated protein kinase 3 [MMK3] from alfalfa) are activated during late anaphase if functional MTs are present [33•,34•]. MTs are
not required to maintain distribution of MMK3 in the cell plate once the PP is established [34•]. Another protein,
Nicotiana protein kinase 1 (NPK1) is similar to upstream kinases in a MAPK chain, referred to as MAPK kinase kinases [35]. NPK1 is expressed in dividing cells [36•] and
complements yeast mutations in homologs to NPK1[35], but its localization patterns relative to the cell plate have not yet been reported. Pending these results, NPK1 in conjunction with p43Ntf6 and MMK3 could be candidate
transducers in a phosphorylation cascade in the cell plate.
Proteins that participate in vesicle targeting are also found in the cell plate. One example is the syntaxin-related pro-tein KNOLLE [27,37]. Syntaxins (also called t-SNARES) are located in target membranes and specifically interact with proteins in arriving vesicles, called v-SNARES [2,38]. Syntaxins in nonplant systems participate in vesicle fusion during cytokinesis [39]. Localization of KNOLLE to vesi-cles and to the equatorial zone suggests it might serve similar recognition and docking functions in the growing cell plate [27,37]. Mutations in KNOLLEcause abnormal cytokinesis consistent with this role: the cell plate is often incomplete, malformed or even absent in mutant cells [37]. In nonplant systems, interactions between target mem-brane and vesicle proteins are mediated by other soluble proteins, including Vacciniavirus complement control pro-tein (VCP) and its homolog in yeast, Cdc48p [40,41]. An Arabidopsis homolog to Cdc48p (AtCDC48) is present in dividing plant cells, localizes to the PP area and is able to complement yeast cdc48mutations [42].
A multi-step process of membrane targeting must be pre-sent in plant cytokinesis: target membranes must be first established before the arriving vesicles can be docked. Next, the cell plate membrane becomes a functional plas-ma membrane, which plas-may occur after cytokinesis is complete. It is interesting that a multi-step process is described ultrastructurally [4], is demonstrated in inhibitor studies [28,43] and is also postulated on the basis of the dis-tribution of typical plasma membrane proteins such as Figure 2
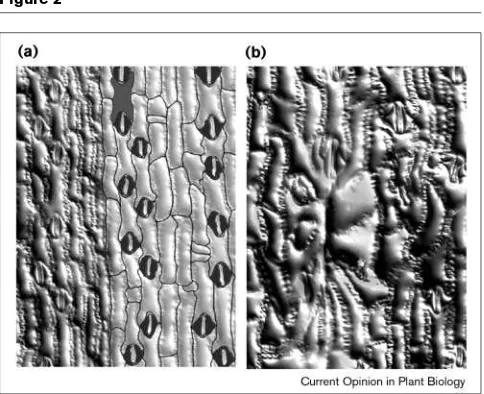
Epidermal cells in a fully-grown maize leaf of (a)a wild-type plant compared with (b)a wty1mutant. (a) The cells in a normal leaf are linear and aligned with the long axis of the leaf blade, as emphasized in the cell outlines in the right of the panel. (b) The mutant leaf has overly enlarged cells in the wart, which result from early events of over-expansion and improper cytokinesis (see also Figure 4).
Figure 3
Simplified view of the pattern of division and expansion in the developing maize leaf primordium. In general, cells in the maize leaf adhere to an inverse pattern of dividing and expanding, particularly below the zone where epidermal cells begin to differentiate (pre-differentiation zone). Here the gradual increase in cell size is due to the rate of expansion exceeding that of division. Orientation of new cross-walls can be observed and quantified in leaf surface impressions made from this region (see Figure 4). Figure reprinted with permission [54•].
Recent cross walls
Cell size
Cell differentiation
Pre-differentiation zone
ATPase, which is absent in the forming cell plate [27]. It will be interesting to find out precisely where and when KNOLLE, for example, is localized in relation to other pro-teins potentially involved in targeting, such as AtCDC48.
Guiding the cell plate to the division site
The nature of the ‘correct site’ at the parental cell wall To accomplish cytokinesis, the PP has to lead the forming cell plate to the right place at about the right time. This intriguing process involves interaction among dynamic MTs of the PP, actin filaments and the cortical division site near the parental cell. The nature of this site is still a mys-tery. Both MTs and actin filaments are present and are required to mark the correct site prior to mitosis when the transient PPB forms [2]. Disassembly of the PPB can be accelerated by injection of CDC2, suggesting cell cycle pro-gression may participate in marking the site [44]. Several hypotheses could explain how the cortical division site is marked. One hypothesis is that marker proteins are phos-phorylated by CDC2 [45]. Alternatively, a localization of ion pumps at the membrane site has been proposed [46]. Another intriguing idea is that the site could be specified by the absence, rather than the presence, of a marker protein. It is interesting that actin remains in the cortex throughout division except at the division site, a position referred to as the actin-depleted zone (Figure 1; [47]). This hypothesis suggests that the absence of scaffolding in the division site balances the PP against the extensive actin scaffolding else-where in the cell. Other than the actin-depleted zone, no persistent structural or biochemical changes have been detected in the cortical division site.Selecting the division site
An explanation is still needed for how the site is chosen, even if specific markers are eventually identified. The division site could indeed be established earlier than just prior to mitosis. For example, the site for any single new cross wall could be influenced by the size and shape of the parent cell and neighboring cells [48,49]. Change in cell wall characteristics could be induced by local strains, caused by differential expansion among interconnected cells [48]. The new division site would then be selected in response to the changed physical parameters of the tissue. Another related view is that the division site is selected on the basis of geometrically correct positions with the cell [50]. Such physical explanations could signal a cell to divide in a particular location, but the biochemical responses elicited by the signal remain unknown.
Spatial signals of cytokinesis were studied recently in onion root cells [51•]. When division is temporarily disrupted by
caffeine, the resulting binucleate cells will eventually adjust and begin dividing again [51•]. Enlarged binucleate cells
generally produced a single PPB at a site predicted from the size and shape of the cell prior to the treatment. These cells would then continue to divide with altered spindle and PP alignments, frequently not related to the most recent PPB position. The results contradict other cases, however, in
which the PPB is absolutely required for correct wall place-ment, such as when a mutant lacks the PPB [52] or when PPs adjust to PPB position after normal spindle rotations [53]. In the experimentally induced binucleate cells, PP position may be signaled by the nucleus or alternatively, by older signals remaining from a prior division. These obser-vations help define the normal parameters of site selection but we clearly do not know all the molecules involved in selecting the division site.
A genetic approach may help explain how the division site is selected. The mutation warty1 (wty1) is providing informa-tion about cell sizes that constrain normal cytokinesis [54•].
The wty1 mutants have cytokinesis defects restricted to groups of cells, producing warts in the leaf blade (Figure 2). Cell sizes can be measured in developing warts near the base of the maize leaf primordium, because of the graduated bal-ance between division and expansion (Figure 3). Epidermal cells in a developing wart initially appear normal with respect to size (Figure 3) and in distribution of cytoskeletal arrays (AW Sylvester, unpublished data). Mutant cells that begin to Figure 4
Leaf surface impressions showing patterns of division and expansion at the base of a developing primordium, in wild-type maize (a,c)and in a
exceed a constant aspect ratio (length to width ratio) at a given position in the primordium will eventually be unable to complete cytokinesis (Figure 3). One explanation for these observations is that over-enlargement prevents normal cytokinesis, possibly by destroying spatial signals within the cell. In these cells, expansion may be prolonged due to an inability to enter mitosis, as wart cells also have endoredupli-cated nuclei with condensed chromosomes (AW Sylvester, unpublished data). Apparently, PP are able to form in warts but appear to lack directional information. Ongoing analysis of the gene and its product will help clarify the role of WTY1 in site selection.
Stabilizing and guiding the phragmoplast
The same proteins may be involved in selecting and also guiding the PP to the division site. For example, actin patches were observed in the actin-depleted zone in dividing cells [47]. Actin filaments emanating from the PP could attach to these patches left behind when the PPB disappears [47,55]. The PP could thus be teth-ered to the division site by the same proteins that originally established the site. Injected profilin, which effectively binds monomeric actin, disrupts cytokinesis, potentially by abolishing an actin-based mechanical support to the forming cell plate [55]. Similarly, caf-feine, which is thought to inhibit cytokinesis by disrupting necessary Ca2+gradients, degrades actin
fila-ments at the leading edge of the cell plate [43]. Consequently, the cell plate arrives at the wrong loca-tion, if at all, and is often incomplete. It is reasonable to conclude that one role of actin is in the guidance of the PP to the division site [55].
The PP and cell plate may also be stabilized internally by actin. In Cliviaendosperm cells, actin filaments are initial-ly interspersed among spindle MTs but soon begin to shorten and polymerize in the equatorial zone, eventually interdigitating among the MTs of the PP (Figure 1; [56•]).
These shorter actin filaments are also associated with a vinculin-like protein [56•]. Vinculin is an actin-associated
protein known to moderate actin–membrane connections [57]. It is possible, therefore, that actin and associated pro-teins, such as vinculin, could be binding the vesicular network into a stable structure, while also guiding it to the division site.
Analysis of the discordia(dcd) mutations dcd1and dcd2may lead us to the molecules that participate in PP guidance [58••]. The dcd mutations cause abnormal growth of cells
that undergo asymmetric cell division in the leaf epider-mis, such as the subsidiary cells in the stomatal complex. Disruption of cell shape is preceded by improperly posi-tioned cell walls, caused by misguided PPs. Actin disruption by cytochalasin phenocopies the mutant defect and the mutant phenotype is also exacerbated by cytocha-lasin treatment. These results suggest that DCD may function in either residual marking of the division site and/or in successful guidance of the PP to the site.
Even if actin proves to be a structural guide, other pro-teins may participate in the process. Clues are coming from another maize gene, tangled1(tan1) [59,60••]. Mutant
tan1 leaves have disorganized cells compared with the well-oriented cells in the normal maize leaf. Cell disorga-nization is attributed partly to the inability of mutant cells to divide in a particular longitudinal orientation (parallel to the leaf axis). Mutant tan1cells also lack the normal dis-trubution of longitudinally oriented PPB, demonstrating the protein is directly or indirectly required for specifying the longitudinal division plane. Furthermore, TAN1 appears to be necessary for proper PP guidance: all PPs in the mutant are slightly disoriented compared with those in normal cells, including those that are presumed to give rise to transversely oriented cell walls. These results are particularly intriguing because they point to the potential for post-cytokinesis adjustments in wall position: cells that have slightly oblique PPs may expand differentially and very slightly, thereby adjusting the new cross wall to a more typical transverse orientation. Preliminary reports suggest the gene may encode a protein with hydrophobic-ity characteristics of a cell-wall protein [61]. Completed analysis of the gene and its protein will be very interest-ing, as will an evaluation of the localization of TAN1 relative to PP and PPBs.
Impact of division ‘decisions’ on the plant body
Division orientations of individual cells vary in their impact on the plant body. For example, morphogenesis of some leaves does not require carefully orchestrated cell divisions [1,59]. On the other hand, some roots have stereotyped division patterns that result in proper disposi-tion of cell layers, required for the acquisidisposi-tion of cell identities [62]. The impact of cytokinesis defects depends on the organ, the developmental stage and the nature of the gene involved. Currently, the phenotypes of mutants that affect cytokinesis reflect this variability. Some of the genes identified are probably redundant, others are direct-ly involved in the mechanics of cytokinesis and others may play a more indirect role in division and expansion. The remainder of this review will evaluate these mutants based on the severity of their phenotype.Fatal divisions
Many mutants with defects in cytokinesis were identified in genetic screens of seedling-lethal embryo-defective mutants [63]. The cyt1mutant of Arabidopsis has defective cross walls and excessive accumulation of callose, evident early in embryogenesis [64••]. Interfering with cellulose
synthesis can phenocopy the mutant, suggesting the gene plays a role in wall production during cytokinesis [64••].
cross walls are initiated but not completed, mimicking the effect of caffeine on cytokinesis.
The gnom/emb30mutation appears to affect all aspects of cell division and expansion. Most gnom mutant cells are able to complete cytokinesis normally, but are disoriented from the first zygotic division [67]. The EMB30/GNOM protein shares a domain with a secretory protein in yeast called Sec7p [68]. Other regions of the protein are similar to a non-essential yeast protein Yec2p [67]. Although the precise role of the protein is not known, the pleiotropic effects of the mutation may be due to generalized defects in secretion or vesicle function, rather than just secretion during cell plate formation.
Differentiation of specific cell types sometimes requires a highly regulated pattern of division [1,69]. Mistakes fre-quently cannot be tolerated in these types of differentiation divisions. One example is in the required asymmetric division that yields polarized cells. Pollen grains will differentiate appropriately only if the first mitotic cross wall is deposited asymmetrically. Incomplete cytokinesis, as seen in gem1 mutants, disrupts differentia-tion of the vegetative and generative cells [70•]. Similarly,
asymmetric cell division precedes differentiation of the layered Arabidopsisroot, which is prevented in scr mutants [71]. In this case, the division alteration is more likely due to a response to a change in positional information rather than a direct effect on cytokinesis. Even so, mutations such as scr may provide information about how these sig-nals are read by cells, an important aspect of the spatial regulation of division [69].
Near fatal divisions
Drastic alterations in cell division may have only partial effects on development. For example, the fass mutants in Arabidopsishave defects in cytokinesis that result in abnor-mally shaped cells [72]. Organ morphogenesis is also severely altered, with plants acquiring a short stubby appearance, but tissues are distributed normally. These mutants also lack the normal PPB, suggesting the gene may be involved in selecting and/or marking the division site [52]. The ton/fass mutants, among others, show that tight spatial regulation of cytokinesis is not necessary for basic tissue differentiation.
Division effects may vary based on whether a gene is required throughout development or in all cells. The tso1 mutants, for example, show cytokinesis defects only in flo-ral organs and ovules [73,74]. In floflo-ral meristems and sepals, cross walls are incomplete and nuclei endoreduplicated, similar to the cytokinetic defects in plants with knolle, keule, cydand wty1mutations. Developmental arrest of ovules in tso1 mutants appears to be due to abnormal and uncoordi-nated cell expansion, a good example of the same gene being required for division or expansion, depending on the organ [73]. Similarly, wty1mutants show altered cytokinesis, but only in leaf blade cells [54•]. If these are all null alleles,
organ specificity of mutant defects suggests either redun-dant functions of the genes in other organs or that the specific controls of division may vary in different organs.
Spatially adjustable divisions
Several mutants have problems with cytokinesis but show quite normal development. Loss of spatial information by altered cytokinesis may be corrected in a growing organ by adjusting the direction or rate of cell expansion or increas-ing the rate of cell division. For example, tan1 mutants have normally shaped leaves and organs, despite incorrect-ly placed cross walls. Cells in tan1 mutants that are experimentally forced to expand more, by growing in dark or low-light conditions, show corrected orientations of cell walls (M Mitkovski, AW Sylvester, unpublished data). Cell adjustments are also seen in wty1 mutants: improper growth of warts is balanced by neighboring cells, which divide more as the leaf is growing [54•]. This neighborly
behavior of cells is consistent with the idea that the emerg-ing organ shape prevails as a signal for the spatial regulation of cell division during morphogenesis of leaves. The idea requires that cells respond together and commu-nicate locally, via regulated plasmodesmatal trafficking [75] or other means of intercellular communication. MTs and cellulose microfibrils, which together direct oriented cell expansion, are observed to be co-aligned in cell groups [49,60••,76], consistent with other observations that
neigh-boring cells grow in coordinated patterns [49]. Mutants, such as tan1 and wty1, provide information about the capacity of cells to adjust within a given framework. Such adjustments suggest that the selection of the division site itself may be flexibly regulated.
Conclusions
The steps of cytokinesis are integrated so that a growing cell plate is guided to a pre-established division site. Several proteins are known that probably contribute to the membranous construction of the cell plate, including phragmoplastin and KNOLLE. Potential phosphoryla-tion requirements in the cell plate will be understood as more kinases are identified. Also, other proteins that might be involved specifically in vesicle targeting, such as CDC48, will provide information about the interac-tions between soluble and membrane-bound proteins. There are several phases in the construction and growth of the cell plate. In the future, it will be interesting to understand how these vesicle proteins may behave uniquely in the cell plate.
is also likely to be responsible for unifying the cell plate and for providing internal as well as lateral support for the PP. Actin may well serve as a scaffolding upon which the PP is poised as it extends radially to the division site. The mech-anism of guiding the PP to its division site is just beginning to unfold, as new mutants are found that influence this process. Mutations such dcd1and dcd2are likely to provide key information about PP guidance.
One outcome of cytokinesis is that two daughter cells are initially positioned relative to neighbors and to the origi-nal parent cell. On the basis of mutant phenotypes, the impact of this division ‘decision’ on subsequent develop-ment depends on the developdevelop-mental stage, the type of protein required, or on the extent of genetic redundancy. Some mutants, such as tan1and wty1can adjust for cytoki-nesis defects, presumably by expanding or dividing differently to compensate for the loss of spatially correct information. In other cases, a correct cell division orienta-tion may be required prior to a critical differentiaorienta-tion step, so that a spatially defective cytokinesis cannot be tolerat-ed, as in the gem1 mutant. Finally, seedling-lethal mutations, such as cyt1, knolle, keule, cydmay cause the loss of some essential function in cytokinesis itself. While the mechanics of cytokinesis is probably shared for different cells in different organs, it is possible that the means of selecting the division site may prove to be variable. In the long term, the question of how a division site is selected should be considered in the context of the entire growing tissue. The interconnections of plant cells suggest that any single division ‘decision’ influences neighboring cells biochemically and ultimately physically. Continued char-acterization of mutants, and molecular analysis of the genes involved, will help to clarify both the mechanics and the determinants of cytokinesis.
Acknowledgements
I wish to thank Jim Reynolds for contributions at all levels to this work. I thank Miso Mitkovski for micrographs and the US Department of Agriculture and the National Science Foundation—Experimental Program for Stimulating Competitive Research for financial support. This review is dedicated to the memory of Professor Paul Green, a source of inspiration and insight.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Sylvester AW, Smith L, Freeling M: Acquisition of identity in the
developing leaf.Annu Rev Cell Dev Biol 1996, 12:257-304.
2. Assaad FF, Mayer U, Lukowitz W, Juergens G: Cytokinesis in
somatic plant cells.Plant Physiol Biochem1997, 35:177-184.
3. Fowler JE, Quatrano RS. Plant cell morphogenesis: plasma
membrane interactions with the cytoskeleton and cell wall.Annu
Rev Cell Dev Biol1997, 13:697-743.
4. Samuels AL, Giddings TH, Staehelin LA: Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher
plants.J Cell Biol1995, 130:1345-1357.
5. Staehelin LA, Hepler PK: Cytokinesis in higher plants.Cell1996,
84:821-824.
6. Mironov V, De Veylder L, Van Montagu M, Inze D: Cyclin-dependent
kinases and cell division in plants-the nexus.Plant Cell1999,
11:509-522.
7. Franklin AE, Cande WZ: Nuclear organization and chromosome
segregation. Plant Cell1999, 11:523-534.
8. Wymer C, Lloyd C: Dynamic microtubules: implications for cell
wall patterns.Trends Plant Sci1996, 1:222-228.
9. Cosgrove DJ: New genes and new biological roles for expansins. Curr Opin Plant Biol2000, 3:73-78.
10. Yasuhara H, Sonobe S, Shibaoka H: Effects of brefeldin A on the
formation of the cell plate in tobacco BY- 2 cells.Eur J Cell Biol
1995, 66:274-281.
11. Hardham AR, Gunning BES: Some effects of colchicine on
microtubules and cell division in roots of Azolla pinnata.
Protoplasma1980, 102:31-51.
12. Thyberg J, Moskalewski S: Role of microtubules in the organization
of the Golgi complex.Exp Cell Res1999, 246:263-279.
13. Yasuhara H, Sonobe S, Shibaoka H: Effects of taxol on the development of the cell plate and of the phragmoplast in tobacco
BY-2 cells.Plant Cell Physiol1993, 34:21-29.
14. Asada T, Collings D: Molecular motors in higher plants.Trends Plant Science1997, 2:29-37.
15. Asada T, Kuriyama R, Shibaoka H: TKRP125, a kinesin-related protein involved in the centrosome-independent organization of
the cytokinetic apparatus in tobacco BY-2 cells.J Cell Sci1997,
110:179-189.
16. Song H, Golovkin M, Reddy AS, Endow SA: In vitromotility of
AtKCBP, a calmodulin-binding kinesin protein of Arabidopsis.
Proc Natl Acad Sci USA1997, 94:322-327.
17. Narasimhulu SB, Reddy AS: Characterization of microtubule
• binding domains in the Arabidopsis kinesin-like calmodulin
binding protein.Plant Cell1998, 10:957-965.
The motor domain of the kinesin-related calmodulin binding protein, KCBP, is shown to bind microtubules in cosedimentation assays with the purified protein. Two MT-binding domains are identified in KCBP, one near the car-boxyl terminus and one near the amino terminus. The protein could thus be involved in bundling MTs. Evidence for the function of this protein in orga-nizing MTs is thus growing.
18. Smirnova EA, Reddy AS, Bowser J, Bajer AS: Minus end-directed
• kinesin-like motor protein, Kcbp, localizes to anaphase spindle
poles in Haemanthusendosperm.Cell Motil Cytoskeleton1998,
41:271-280.
KCBP distribution is observed in endosperm cells during mitosis and cytoki-nesis. Interesting shifts in localization patterns are seen during a transition through telophase. KCBP shifts from an association with the spindle poles at the end of mitosis to the equatorial zone. KCBP is eventually localized along the phragmoplast MTs, but also to the nuclear membrane early in telophase and to the cell plate in late telophase. Slight differences in KCBP distribution with BY-2 cells may be due to unique properties of the cellular-izing endosperm.
19. Liu B, Cyr RL, Palevitz BA: A kinesin-like protein, KatAp, in the cells of Arabidopsis and other plants.Plant Cell1996, 8:119-132. 20. Compton DA: Focusing on spindle poles.J Cell Sci1998,
111:1477-1481.
21. Kakimoto T, Shibaoka H: Cytoskeletal ultrastructure of
phragmoplast-nuclei complexes isolated from cultured tobacco
cells.Protoplasma1988, 2:95-103.
22. Parke J, Miller C, Anderton BH: Higher plant myosin heavy-chain identified using a monoclonal antibody.Eur J Cell Biol1986, 41:9-13. 23. Asada T, Shibaoka H: Isolation of polypeptides with
microtubule-translocating activity from phragmoplasts of tobacco BY-2 cells. J Cell Sci1994, 107:2249-2257.
24. Gu XJ, Verma DPS: Phragmoplastin, a dynamin-like protein
associated with cell plate formation in plants.EMBO J1996,
15:695-704.
25. Park JM, Kang SG, Pih KT, Jang HJ, Piao HL, Yoon HW, Cho MJ, Hwang I: A dynamin-like protein, ADL1, is present in membranes
as a high-molecular-mass complex in Arabidopsis thaliana.Plant
Physiol1997, 115:763-771.
27. Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jurgens G: The ArabidopsisKNOLLE protein is a
cytokinesis-specific syntaxin.J Cell Biol1997, 139:1485-1493.
28. Gu X, Verma DP: Dynamics of phragmoplastin in living cells during cell plate formation and uncoupling of cell elongation from the plane of cell division.Plant Cell1997, 9:157-169.
29. Slepnev VI, Ochoa GC, Butler MH, Grabs D, Camilli PD: Role of phosphorylation in regulation of the assembly of endocytic coat
complexes.Science1998, 281:821-824.
30. Sharp DJ, McDonald KL, Brown HM, Matthies HJ, Walczak C, Vale RD, Mitchison TJ, Scholey JM: The bipolar kinesin, KLP61F, cross-links
microtubules within interpolar microtubule bundles of Drosophila
embryonic mitotic spindles.J Cell Biol1999, 144:125-138.
31. Hirt H: Multiple roles of MAP kinases in plant signal transduction. Trends Plant Sci1997, 2:11-15.
32. Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH: Association of mitogen-activated protein kinase with the microtubule
cytoskeleton.Proc Natl Acad Sci USA1995, 92:8881-8885.
33. Calderini O, Bogre L, Vicente O, Binarova P, Heberle-Bors E,
• Wilson C: A cell cycle regulated MAP kinase with a possible role
in cytokinesis in tobacco cells.J Cell Sci1998, 111:3091-3100.
This MAPK from tobacco is activated during late anaphase and the protein localizes to the cell plate. Disruption of MTs with inhibitors prevents activa-tion of the kinase.
34. Bogre L, Calderini O, Binarova P, Mattauch M, Till S, Kiegerl S,
• Jonak C, Pollaschek C, Barker P, Huskisson NS et al.: A MAP kinase is activated late in plant mitosis and becomes localized to the
plane of cell division.Plant Cell1999, 11:101-114.
A MAPK is characterized from alfalfa. The protein appears to be distributed in the region of the cell plate and remains there even as the phragmoplast extends radially.
35. Banno H, Hirano K, Nakamura T, Irie K, Nomoto S, Matsumoto K, Machida Y: NPK1, a tobacco gene that encodes a protein with a domain homologous to yeast BCK1, STE11, and Byr2 protein
kinases.Mol Cell Biol1993, 13:4745-4752.
36. Nakashima M, Hirano K, Nakashima S, Banno H, Nishihama R,
• Machida Y: The expression pattern of the gene for NPK1 protein kinase related to mitogen-activated protein kinase kinase kinase (MAPKKK) in a tobacco plant: correlation with cell proliferation. Plant Cell Physiol1998, 39:690-700.
An upstream MAPK (identified as a MAPK kinase kinase) was found in tobacco and is expressed in dividing cells. Subcellular localization is still needed to clarify whether or not this protein is part of a phosphorylation cas-cade in the cell plate or phragmoplast.
37. Lukowitz W, Mayer U, Jurgens G: Cytokinesis in the Arabidopsis
embryo involves the syntaxin-related KNOLLE gene product.Cell
1996, 84:61-71.
38. Robinson DG, Hinz G, Holstein SE: The molecular characterization
of transport vesicles.Plant Mol Biol1998, 38:49-76.
39. Jantsch-Plunger V, Glotzer M: Depletion of syntaxins in the early
Caenorhabditis elegansembryo reveals a role for membrane
fusion events in cytokinesis.Curr Biol1999, 9:738-745.
40. Mellman I: Enigma variations: protein mediators of membrane
fusion.Cell1995, 82:869-872.
41. Frohlich KU, Fries HW, Rudiger M, Erdmann R, Botstein D, Mecke D:
Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene
expression.J Cell Biol1991, 114:443-453.
42. Feiler HS, Desprez T, Santoni V, Kronenberger J, Caboche M, Traas J:
The higher plant Arabidopsis thaliana encodes a functional
CDC48 homologue which is highly expressed in dividing and
expanding cells.EMBO J1995, 14:5626-5637.
43. Valster AH, Hepler PK: Caffeine inhibition of cytokinesis: effect on
the phragmoplast cytoskeleton in living Tradescantiastamen hair
cells.Protoplasma1997, 196:155-166.
44. Hush J, Wu L, John PC, Hepler LH, Hepler PK: Plant mitosis promoting factor disassembles the microtubule preprophase
band and accelerates prophase progression in Tradescantia.Cell
Biol Int Rep1996, 20:275-287.
45. Cleary AL: F-actin redistributions at the division site in living
Tradescantiastomatal complexes as revealed by microinjection of
rhodamine–phalloidin.Protoplasma1995, 185:152-165.
46. Cyr R: Calcium/calmodulin affects microtubule stability in lysed
protoplasts.J Cell Sci1991, 100:311-317.
47. Cleary AL, Gunning BES, Wasteneys GO, Hepler PK: Microtubule
and F-actin dynamics at the division site in living Tradescantia
stamen hair cells.J Cell Sci1992, 103:977-988.
48. Green PB: Transductions to generate plant form and pattern: an
essay on cause and effect.Ann Bot1996, 78:269-281.
49. Sylvester AW, Williams MH, Green PB: Orientation of cortical microtubules correlates with cell shape and division direction: immunofluorescence of intact epidermis during development of
Graptopetalum paraguayensis.Protoplasma1989, 153:91-103. 50. Flanders DJ, Rawlins DJ, Shaw PJ, Lloyd CW: Nucleus-associated
microtubules help determine the division plane of plant epidermal cells: avoidance of four way junctions and the role of cell
geometry.J Cell Biol1990, 110:1111-1122.
51. Gimenez-Abian MI, Utrilla L, Canovas JL, Gimenez-Martin G,
• Navarrete MH, De la Torre C: The positional control of mitosis and
cytokinesis in higher-plant cells.Planta1998, 204:37-43.
Binucleate wheat cells are produced by caffeine treatment and subsequent patterns of phragmoplast arrangement are recorded in cells of different sizes. The authors conclude that PP could form without respect to the PPB in some cases, suggesting pre-existing spatial cues may dictate cell wall position under certain circumstances.
52. Traas J, Bellini C, Nacry P, Kronenberger J, Bouchez D, Caboche M:
Normal differentiation patterns in plants lacking microtubular
preprophase bands.Nature1995, 375:676-677.
53. Palevitz BA: Division plane determination in guard mother cells of
Allium: video time-lapse analysis of nuclear movements and
phragmoplast rotation in the cortex.Dev Biol1986, 117:644-654.
54. Reynolds JO, Eisses JF, Sylvester AW: Balancing division and
• expansion during maize leaf morphogenesis: analysis of the
mutant, warty-1.Development1998, 125:259-268.
Cells with wty1 mutations undergo faulty cytokinesis once the cells have begun to over-expand. The results suggest that one spatial cue for the divi-sion site may be appropriate length to width or volume ratio in the cell. In addition, neighboring cells in the wty1 mutant, which can look normal, appear to adjust to the defect by dividing more.
55. Valtzer AH, Pierson ES, Valenta R, Hepler PK, Emons AMC: Probing the plant actin cytoskeleton during cytokinesis and interphase by
profilin microinjection.Plant Cell1997, 9:1815-1824.
56. Endle MC, Stoppin V, Lambert AM, Schmit AC: The growing cell
• plate of higher plants is a site of both actin assembly and
vinculin-like antigen recruitment.Eur J Cell Biol1998, 77:10-18.
The distribution of actin and an associated vinculin-like protein is described in endosperm cells of Clivia. Short actin filaments are seen in the center of the PP and co-localize with vinculin. The authors suggest the proteins are important in maintaining and perhaps guiding the PP during cytokinesis. 57. Johnson RP, Craig SW: F-actin binding site masked by the
intramolecular association of vinculin head and tail domains. Nature1995,373:261-264.
58. Gallagher K, Smith LG: discordiamutations specifically misorient
•• asymmetric cell divisions during development of the maize leaf
epidermis.Development1999, 126:4623-4633.
These mutants show an interesting and informative phenotype. Asymmetric divisions that occur during differentiation of the epidermis are affected so that cytokinesis is disrupted and cells expand in abnormal orientations. Normally positioned PPB are present, predicting the correct site for the impending new wall. PP lose direction relative to the site, however, sug-gesting that they are not guided properly or that residual information mark-ing the site is lost. Particularly intrigumark-ing is the fact that actin filament disruption can phenocopy the mutants. The results implicate the genes in a PP guidance process that may require actin or associated proteins. 59. Smith LG, Hake S, Sylvester AW: The tangled-1 mutation alters cell
division orientations throughout maize leaf development without
altering leaf shape.Development1996, 122:481-489.
60. Cleary AL, Smith LG: The Tangled1gene is required for spatial
•• control of cytoskeletal arrays associated with cell division during
maize leaf development.Plant Cell1998, 10:1875-1888.
61. Miller D, Hable W, Gottwald J, Ellard-Ivey M, Demura T, Lomax T, Carpita N: Connections: the hard wiring of the plant cell for
perception, signaling, and response.Plant Cell1997, 9:2105-2117.
62. Berger F, Hung CY, Dolan L, Schiefelbein J: Control of cell division in
the root epidermis of Arabidopsis thaliana.Dev Biol1998,
194:235-245.
63. Meinke DW: Perspectives on genetic analysis of plant
embryogenesis.Plant Cell1991, 3:857-866.
64. Nickle TC, Meinke DW: A cytokinesis-defective mutant of
•• Arabidopsis(cyt1) characterized by embryonic lethality,
incomplete cell walls, and excessive callose accumulation.Plant J
1998, 15:321-332.
The cyt1mutant shows lethal defects in cytokinesis that result in abnormal cross-walls and excessive accumulation of callose — a component of the forming cell plate. Cellulose synthesis disruption phenocopies the mutant defect, suggesting the gene may be involved in regulating the transition form cytokinesis to cell wall synthesis.
65. Assaad FF, Mayer U, Wanner G, Jurgens G: The KEULE gene is
involved in cytokinesis in Arabidopsis.Mol Gen Genet1996,
253:267-277.
66. Liu CM, Johnson S, Wang TL: cyd, a mutant of pea that alters
embryo morphology is defective in cytokinesis.Dev Genet1995,
16:321-331.
67. Busch M, Mayer U, Jurgens G: Molecular analysis of the
Arabidopsispattern formation of gene GNOM: gene structure
and intragenic complementation.Mol Gen Genet1996,
250:681-691.
68. Shevell DE, Leu WM, Gillmor CS, Xia G, Feldmann KA, Chua NH:
EMB30 is essential for normal cell division, cell expansion, and
cell adhesion in Arabidopsis and encodes a protein that has
similarity to Sec7.Cell 1994, 77:1051-1062.
69. Scheres B, Heidstra R: Digging out roots: pattern formation, cell
division and morphogenesis in plants.Curr Top Dev Biol1999,
45:207-247.
70. Park SK, Howden R, Twell D: The Arabidopsis thaliana
• gametophytic mutation gemini pollen1disrupts microspore
polarity, division asymmetry and pollen cell fate.Development
1998, 125:3789-3799.
The gem1mutation is an example of a differentiation process that requires the correct spatial information of an asymmetric division. Cytokinesis is dis-rupted in the developing pollen grain so that essential asymmetries are not carried forward to permit specification of the vegetative and generative cells. 71. Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y,
Freshour G, Hahn MG, Feldmann KA, Benfey PN: The SCARECROW gene regulates an asymmetric cell division that is essential for
generating the radial organization of the Arabidopsisroot.Cell
1996, 86:423-433.
72. Torres-Ruiz RA, Jurgens G: Mutations in the FASS gene uncouple
pattern formation and morphogenesis in Arabidopsis
development.Development1994, 120:2967-2978.
73. Hauser BA, Villanueva JM, Gasser CS: ArabidopsisTSO1 regulates directional processes in cells during floral organogenesis. Genetics1998, 150:411-423.
74. Liu Z, Running MP, Meyerowitz EM: TSO1 functions in cell division
during Arabidopsisflower development.Development1997,
124:665-672.
75. Itaya A, Woo YM, Masuta C, Bao Y, Nelson RS, Ding B: Developmental regulation of intercellular protein trafficking through plasmodesmata
in tobacco leaf epidermis.Plant Physiol1998, 118:373-385.
76. Hardham AR, Green PB, Laag JM: Reorganization of cortical microtubules and cellulose deposition during leaf formation in