Summary Six 25- to 30-year-old slash pine, Pinus elliottii Englm. var. elliottii, trees were inoculated with Ophiostoma minus (Hedgc.) H.P. Sydow, O. ips (Rumb.) Nannf or sterile water. Two, 4 and 6 weeks after inoculation, the lengths of developing lesions and the monoterpene concentration of the necrotic tissue within each lesion were measured. Both sterile and fungal wounding resulted in the development of lesions in the phloem--outer xylem. At both 4 and 6 weeks after inocula-tion, lesions induced by O. minus were significantly larger than lesions induced by O. ips or sterile water, whereas the lesions induced by O. ips and sterile water were similar in size at all sampling periods. At 2, 4 and 6 weeks after inoculation, lesions induced by O. minus had significantly greater concentrations of monoterpenes than lesions induced by O. ips or sterile water. The monoterpene concentration of lesions induced by O. ips was significantly greater than that of lesions induced by sterile water only at the 6-week sampling period. Visual examination of the lesions indicated that O. minus but not O. ips was inhibiting the development of callus tissue, suggesting that the strain of O. ips was either nonpathogenic or avirulent. Keywords: Ceratocystis, monoterpene concentration, Ophios-toma, phloem wounding, Pinus elliottii, xylem wounding.
Introduction
Pine bark beetles (Scolytidae) initiate attacks on trees by boring through the outer bark to the phloem--xylem interface. Simultaneously, spores of their fungal symbionts, most com-monly blue stain fungi of the genus Ophiostoma (formerly Ceratocystis) are introduced into the tree. It is during this initial attack and colonization phase that the beetle--microor-ganism complex encounters the host’s defenses.
In pines, defense against attacks by bark beetles and the fungi they vector includes wound cleansing, wound contain-ment and wound healing (Berryman 1972). The wound con-tainment, or induced response as it is commonly referred to, is initiated in the phloem and sapwood in response to fungal invasion. This response is characterized by a reduction in the amount of water and sugars, and an accumulation of poten-tially toxic monoterpenes and phenolic compounds in the cells of the developing lesion (Reid et al. 1967, Shrimpton 1973,
Lieutier and Berryman 1988). It is hypothesized that this response inhibits the spread of the beetle, the fungus, or both, thereby allowing the development of a new periderm.
Although the induced response is considered to be a gener-alized response to wounding, the intensity of the induced response to beetle-vectored fungi is greater than to a sterile wound. In loblolly pine, Pinus taeda L., Cook and Hain (1985) found that Ophiostoma minus (Hedgc.) H.P. Sydow and an-other fungus, perhaps Ceratocystiopsis ranaculosus Perry and Bridges, which are vectored by the southern pine beetle, Den-droctonus frontalis Zimmermann, induced significantly larger lesions than sterile wounds. They also found that O. minus induced significantly larger lesions than the other fungus indi-cating that the intensity of the response varies among fungal taxa. Paine (1984) made similar observations after inoculating ponderosa pine, Pinus ponderosa Dougl., with O. minus and O. nigrocarpa (Davids.) DeHoog, two fungi vectored by the western pine beetle, D. brevicomis LeConte.
The intensity and speed of induction also vary among indi-vidual trees and appear to be correlated with tree vigor and the tree’s ability to resist beetle attack. For example, grand fir, Abies grandis (D. Don ex Lamb.) Lindl., trees that were resis-tant to attacks by the fir engraver, Scolytus ventralis LeConte, were characterized by more rapid accumulation of resin and formation of callus tissue at the margins of lesions than suscep-tible trees (Berryman 1969, Berryman and Ashraf 1970). Raffa and Berryman (1982, 1983) observed that lodgepole pine, Pinus contorta Dougl., trees that were resistant to western pine beetle attacks were characterized by greater and more rapid accumulation of resin following artificial inoculation with O. clavigerum (Robins.-Jeff.-Davids.) Harrington than sus-ceptible trees. The monoterpene concentration of lesion tissue averaged 90.0 mg g−1 compared with 13.4 mg g−1 in suscepti-ble trees. Cook and Hain (1987) found that the size of lesions produced following inoculation of shortleaf pine, Pinus echi-nata Mill., with O. minus was related to the tree’s ability to resist southern pine beetle attacks. Trees that resisted attack had significantly shorter lesions than trees that were success-fully attacked.
These studies indicate that the intensity of the host response to fungal infection differs among species of bark-beetle-vec-tored fungi, and that the intensity and speed of induction differ
Characterization of the induced response of slash pine to inoculation
with bark beetle vectored fungi
MICHAEL P. POPP,
1,2JON D. JOHNSON
1and MARK S. LESNEY
11 Department of Forestry, University of Florida, Gainesville, FL 32611, USA
2 Current address: Department of Ophthalmology, Box 100284 JHMHC, University of Florida, Gainesville, FL 32610, USA
Received September 20, 1993
among individual trees. In particular, the results of Cook and Hain (1987) suggest that, in some cases, large lesion size and its accompanying responses are not correlated with resistance but are evidence of increased damage as a result of pathogen spread. Because the intensity and speed of the induction proc-ess appear to be correlated with the capacity for succproc-essful defense, identification of the mechanisms that regulate the induced response may lead to the ability to alter tree defense and to select for resistant trees. Therefore, we have charac-terized the induced response in slash pine, Pinus elliottii Englm. var. elliottii, the predominant pine species in Florida.
Materials and methods
Inoculation with O. minus and O. ips
Isolates of O. minus (American Type Culture Collection 15271) and O. ips (Rumb.) Nannf were obtained from the Forest Service Laboratory in Pineville, LA, and subcultured in petri plates on 2% potato dextrose agar (PDA) until ascocarps developed. Five ml of sterile water was then added to each petri plate and, after scarifying the agar surface with a sterile loop, the spores were isolated by passing the suspension through a 61 µm filter. Spores were counted with a hemocytometer.
Six 25- to 30-year-old slash pine trees, located on a site near Windsor, FL, were inoculated during April 1987. Before in-oculation, the outer bark was shaved smooth and sprayed with 70% isopropyl alcohol. Three sets of three wounds to the xylem (spaced equidistantly around the circumference of each tree) were made on each tree at breast height with a sterile 2.54-cm arch punch. Phloem tissue from the outer bark--phloem plug was removed, wrapped separately in aluminum foil and placed on dry ice for transport to the laboratory where all phloem samples were stored at −70 °C. Aliquots (0.5 ml) of O. minus spore suspension (3.6 × 106 spores ml−1), O. ips (3.6 × 106 spores ml−1) or sterile water were randomly applied to each set of three wounds with a tuberculin syringe. The outer bark plug was then replaced and covered with tape to prevent contamination.
Sample collection
Trees were sampled 2, 4 and 6 weeks after inoculation. At each sample time, the same randomly selected face of each tree was sampled by peeling away the outer bark and exposing the developing lesions. Total lesion length was measured and the proportion of the original wound covered by callus tissue was visually estimated. The necrotic resin-soaked lesion was carved from the tree, wrapped in aluminum foil and placed on dry ice for transport to the laboratory where the samples were stored at −70 °C.
Monoterpene analysis
Phloem samples were thawed, finely chopped and extracted with pentane containing a known amount of p-cymene as an internal standard for monoterpenes. After 24 h, pentane was decanted into a vial and the remaining woody material was dried at 65 °C to constant weight. Pentane extracts were
ana-lyzed with a Hewlett-Packard 5890 gas chromatograph equipped with an Alltech 25 m superox capillary column (0.25 ml internal diameter and 0.2 µm film) and a flame ioni-zation detector. Oven temperature was held at 55 °C for 5 min and then raised (25 °C min−1) to a final temperature of 160 °C to burn off any residual sample. Individual monoterpenes were identified by comparing the retention time of unknown peaks with the retention time of monoterpene standards. The identi-ties of suspected monoterpene peaks were verified by mass spectrometry.
The amounts of the six major monoterpenes present in slash pine (in order of chromatographic retention: α-pinene, cam-phene, β-pinene, myrcene, limonene and β-phellandrene) were determined by converting the area under the corresponding peak to p-cymene equivalents. Para-cymene equivalents were converted to milliliters of monoterpene as described by Raffa and Steffeck (1988). Volumes of the six monoterpenes were summed and expressed as milliliters of total monoterpenes per gram of dry phloem tissue.
Inoculation with additional fungal symbionts
Cultures of Leptogaphium terebrantis Barras and Perry (C326), O. minus (C220) and O. nigrocarpa (C283) were obtained from Dr. T. Harrington (Department of Plant Pathol-ogy, Iowa State University, Ames, IA). Subsamples of these cultures were placed on PDA plates and allowed to grow until sporulation.
Six 15- to 18-year-old slash pine trees, located on a site near Windsor, FL, were inoculated during May 1990. Before inocu-lation, the outer bark was shaved smooth and sprayed with 70% isopropyl alcohol. One set of four wounds to the xylem (spaced about 5 cm apart) was made on the north face of each tree at breast height with a sterile 2.54-cm arch punch. A 0.6-cm diameter disk was cut from the agar plates of each of the three different fungi and placed in the wound. A sterile wound without an agar disk served as a control. Phloem tissue from the outer bark--phloem plug was removed and the outer bark plug was replaced and covered with tape to prevent contamination. During April 1991, the outer bark from inocu-lated trees was shaved to expose the wounds. The vertical length of each wound was measured. In addition, qualitative observations of resin color, degree of resin crystallization and degree of tissue maceration were made for each wound.
Statistical analysis
Results
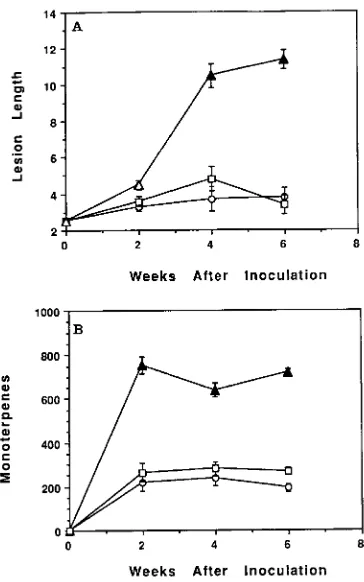
Unwounded phloem tissue was turgid, white and contained less than 1.0 µl of total monoterpenes per gram dry weight (Figure 1). Both sterile and fungal wounding resulted in the development of a lesion in the phloem tissue around the origi-nal wound, but the intensity of this induced response differed among treatments. Two weeks after inoculation with sterile water, a 0.8-cm lesion had developed around the original 2.54-cm wound, and the monoterpene concentration of this tissue was 220 µl g−1, a 200-fold increase above that of un-wounded phloem (Figure 1). Neither lesion size nor monoter-pene concentration of the lesion tissue increased appreciably after the second week (Figure 1). Six weeks after inoculation, the water-inoculated wound on all sample trees had begun to heal as indicated by the presence of callus tissue covering more than 50% of the original wound.
Two weeks after inoculation with O. minus, a 2-cm elliptical lesion had developed above and below the original wound. The monoterpene concentration of the lesion tissue increased to 754 µl g−1. In contrast to the lesion induced by sterile water, the lesion induced by O. minus continued to enlarge through-out the 6-week study and was significantly greater in length at the 2- and 4-week sampling periods than the lesions induced
by inoculation with sterile water (Figure 1). Although there was an increase in the size of the lesions induced by O. minus after Week 2, there was not a concomitant increase in the monoterpene concentration of the lesion tissue; however, the monoterpene concentration of the lesions induced by O. minus remained significantly higher than that of lesions induced by sterile water (Figure 1). None of the O. minus inoculated wounds had any callus tissue over the original wound at 6 weeks after inoculation.
The size of lesions induced by O. ips was not significantly different from that of lesions induced by sterile water (Fig-ure 1). The monoterpene concentration of lesions induced by O. ips was greater than that of unwounded phloem but was only significantly greater than that of lesions induced by sterile water at Week 6. The monoterpene concentration of lesions induced by O. ips was significantly lower than that of lesions induced by O. minus on all sample dates. The lesions induced by O. ips were similar to the lesions induced by sterile water in their ability to heal. Six weeks after inoculation, 87% of the original wounds had callus tissue present that covered 50% or more of the original wound.
In the second study, O. minus and L. terebrantis, but not O. nigrocarpum, induced lesions that were significantly larger than the lesions induced by sterile water (Table 1). In addition, the lesions induced by O. minus and L. terebrantis differed in appearance from the lesions induced by O. nigrocarpum and sterile water. The lesions induced by O. minus and L. terebran-tis exhibited tissue maceration and tended to be inundated with yellow resin, whereas the lesions induced by O. nigrocarpum and sterile water had little or no tissue maceration and the resin tended to be white and crystallized. Extraction of the lesions indicated that the resin-soaked tissue extended radially into the xylem tissue a greater distance in the O. minus- and L. tere-brantis-treated wounds than in the O.nigrocarpum- and ster-ile-water-treated wounds. In many instances, the resin-soaked tissues also extended upward beyond the visible wound so that part of the lesion was not visible by simply shaving off bark and phloem. This type of resin-soaking pattern was not present in the previous study.
Figure 1. (A) Mean lesion length (cm) and (B) mean total monoterpene concentration (µl g−1) of induced lesions in 25--30-year-old slash pine following inoculation with either sterile water (s), Ophiostoma minus
spores (m) or Ophiostoma ips spores (h). The same shading pattern
within a week indicates no significant difference between means based on Duncan’s new multiple range test (P = 0.05). Bars represent mean
± the standard error of the mean.
Table 1. Mean total lesion length (cm) in 15--18-year-old slash pine 1 year after inoculation with either sterile water, Ophiostoma minus, Ophiostoma nigrocarpum or Leptographium terebrantis.
Inoculum Lesion length1
Sterile water 4.60 a
Ophiostoma nigrocarpum 4.90 a
Leptographium terebrantis 9.68 b
Ophiostoma minus 12.05 b
1 The same letter following the mean indicates no significant
Discussion
The response of slash pine to bark-beetle-vectored fungi is similar to that observed in other conifers, where wounding and inoculation with bark-beetle-vectored fungi induce the pro-duction of monoterpene-soaked lesions. The response is also consistent with the conclusion that the size and monoterpene concentration of induced lesions increase as the pathogenicity of the fungal taxon increases (Paine 1984, Cook and Hain 1985, 1986, Owen et al. 1987, Pain and Stephen 1987). Large lesions with a high monoterpene concentration may indicate a more active defense response or greater resistance to the bee-tle--fungus complex (Raffa and Berryman 1982, 1983, Paine 1984, Cook and Hain 1985, 1986, Owen et al. 1987, Paine and Stephen 1987). In many cases, beetle resistance has been presumed by studying the response of the tree to beetle-vec-tored fungi, as measured by large lesion size and high monter-pene concentration of the lesion tissue, without studying subsequent beetle attack (Paine 1984, Cook and Hain 1985, 1986, Paine and Stephen 1987). However, our data suggest that identical resistance mechanisms are not operating against both the beetle and fungus for all host species.
We found that O. minus induced a larger lesion with a higher monoterpene concentration than sterile water, and it produced a larger and more monoterpene-soaked lesion than O. ips. The only difference between lesions induced by O. ips and those induced by sterile water was that the former had a higher concentration of monoterpenes at Week 6. The finding that lesions induced by sterile water and O. ips, but not by O. minus, began to heal suggests that only O. minus is pathogenic or that the isolate of O. ips we used is avirulent. Owen et al. (1987) and Bennet and Tattar (1988) also found that O. ips displayed an avirulent or nonpathogenic response based on the presence of small lesions with little resin or the presence of callus tissue around the wound. Because callus development, which repre-sents the beginning of wound healing (Berryman 1972), was not present in wounds induced by O. minus, we conclude that large lesions with a high monoterpene concentration reflect greater fungal virulence rather than greater host resistance. This conclusion is also supported by the results of the second study where resin soaking occurred deeper in the sapwood and greater tissue maceration was present following inoculation with the more pathogenic O. minus and L. terebrantis than with O. nigrocarpum (Paine 1984, Owen et al. 1987). In addition, Ballard et al. (1984) found that, in naturally infected lodgepole pine, fungal hyphae initially grew and proliferated radially within the ray parenchyma, but in more advanced infections, hyphae penetrated tracheids and spread longitudinally and occasionally spread to adjacent tracheids. Basham (1970) re-ported that pathogenic isolates of Ophiostoma spp. in loblolly pine penetrated the living sapwood, whereas nonpathogenic isolates did not.
In our research with slash pine and in other research with loblolly pine (Cook and Haine 1985, 1986, Paine and Stephen 1987), the response induced by more than one fungal taxon has been compared in trees that were not subsequently attacked by beetles, or in trees known to differ in host vigor. Thus, what has actually been tested is pathogenicity of the different fungal
taxa on apparently healthy trees. Therefore, in all cases, except where Raffa and Berryman (1982, 1983) clearly showed that large lesion size was correlated with beetle resistance, we contend that large lesions represent fungal virulence and host susceptibility to the fungus rather than a more active resistance response to the beetle--fungus complex. This conclusion is further supported by the fact that Ophiostoma fungi are facul-tative parasites, and at Week 2, the lesion induced by O. minus had a 2.8-fold higher monoterpene concentration than the lesion induced by O. ips. Despite the high concentration of monoterpenes, the development of callus tissue was inhibited and the lesion continued to expand indicating that the monoter-penes were unable to contain O. minus. We conclude that the disparity in the literature among experiments performed on different tree species with different beetle-vectored fungi rep-resents separation of different resistance mechanisms against beetles and fungi. However, in discussion, these mechanisms are conflated due to the assumption of uniform cooperativity between the attacking species in overcoming all defenses, which is yet to be proven and which our results show to be unlikely.
Acknowledgments
We thank Dr. Tom Dean for help with statistical analyses, Drs. Bob Bridges and Tom Harrington for providing fungal isolates, and Dr. John Toth for mass spectrometry analyses. We also thank Drs. Ken Raffa, Tom Harrington and John Davis for reviewing earlier versions of this manuscript. This work was partially supported by U.S. Depart-ment of Agriculture Grant 89-37250-4522 to J.D.J. This is journal series R-03665 of the Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL.
References
Ballard, R.G., M.A. Walsh and W.E. Cole. 1984. The penetration and growth of blue stain fungi in the sapwood of lodgepole pine at-tacked by mountain pine beetle. Can. J. Bot. 62:1724--1729. Basham, H.G. 1970. Wilt of loblolly pine inoculated with blue stain
fungi of the genus Ceratocystis. Phytopathology 60:750--754. Bennet, E.M. and T.A. Tattar. 1988. Blue stain fungi and insect vector
interactions in Japanese black pine and Scots pine mortality. Arboric. J. 12:237--247.
Berryman, A.A. 1969. Responses of Abies grandis to attack by Scolytus ventralis (Coleoptera: Scolytidae). Can. Entomol. 101:1033--1041.
Berryman, A.A. 1972. Resistance of conifers to invasion by bark beetle fungus associations. Bioscience 22:598--601.
Berryman, A.A. and M. Ashraf. 1970. Effects of Abies grandis resin on the attack behavior and brood survival of Scolytus ventralis (Coleoptera: Scolytidae). Can. Entomol. 102:1229--1236. Cook, S.P. and F.P. Hain. 1985. Qualitative examination of the
hyper-sensitive response of loblolly pine, Pinus taeda L., inoculated with two fungal associates of the southern pine beetle, Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). Environ. Entomol. 14:396--400.
Cook, S.P. and F.P. Hain. 1987. Susceptibility of trees to southern pine beetle, Dendroctonus frontalis (Coleoptera: Scolytidae). Environ. Entomol. 16:9--14.
Lieutier, F. and A.A. Berryman. 1988. Preliminary histological inves-tigations of the defense reactions of three pines to Ceratocystis clavigera and two chemical elicitors. Can. J. For. Res. 18:1243--1247.
Owen, D.R., K.Q. Lindahl Jr., D.L. Wood and J.R. Parmeter Jr. 1987. Pathogenicity of fungi isolated from Dendroctonus valens, D. bre-vicomis, and D. ponderosae to pine seedlings. Phytopathology 77:631--636.
Paine, T.D. 1984. Seasonal response of ponderosa pine to inoculation of the mycangial fungi from the western pine beetle. Can. J. Bot. 62:551--555.
Paine, T.D. and F.M. Stephen. 1987. Fungi associated with the south-ern pine beetle: avoidance of the induced defense response in loblolly pine. Oecologia 74:377--379.
Raffa, K.F. and A.A. Berryman. 1982. Physiological differences be-tween lodgepole pines resistant to the mountain pine beetle and associated microorganisms. Environ. Entomol. 11:486--492. Raffa, K.F. and A.A. Berryman. 1983. Physiological aspects of
lodge-pole pine wound responses to a fungal symbiont of the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae). Can. Entomol. 115:723--734.
Raffa, K.F. and R.J. Steffeck. 1988. Computation of response factors for quantitative analysis of monoterpenes by gas-liquid chromatog-raphy. J. Chem. Ecol. 14:1385--1390.
Reid, R.W., H.S. Whitney and J.A. Watson. 1967. Reaction of lodge-pole pine to attack by Dendroctonus ponderosae Hopkins and blue stain fungi. Can. J. Bot. 45:1115--1126.