I would like to thank all current and former lab members for making this lab such an incredible home for me over the past few years. In addition, I would like to thank Sarah Weinstein for carefully proofreading this thesis.
Abstract
Introduction
Herein, we will review the previous literature on the role of O-GlcNAcylation in metabolism and neuronal function, with a specific focus on the role of O-GlcNAcylation in the maintenance of glucose homeostasis in the liver and its role in neuronal signaling and neuroprotection . In the liver, we will outline the role of O-GlcNAsylation in insulin resistance, gluconeogenesis and lipid metabolism.
The Role of O-GlcNAc in Metabolic Homeostasis. Homeostasis
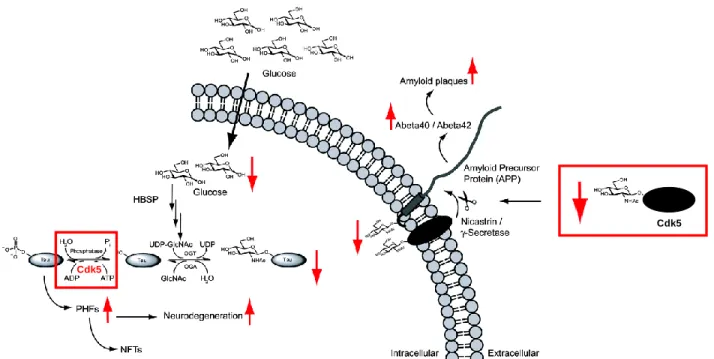
At the organismal level, increased O-GlcNAcylation in ArgP neurons of the hypothalamus may suppress the browning of white fat, which alone (or through other mechanisms) ultimately leads to increased insulin resistance and susceptibility to diet-induced obesity.75. blocking O-GlcNAcylation in adipocytes has been shown to reduce the production of signaling molecules that induce hyperphagia associated with a high-fat diet and promote intrinsic lipolysis.76,77 Taken together, the direct relationship between extracellular glucose concentrations and O-GlcNAcylation of intracellular proteins can have far-reaching and detrimental consequences in T2DM. In fact, several mitochondrial proteins, including many proteins in the electron transport chain and enzymes in the tricarboxylic acid cycle, are regulated by O-GlcNAcylation.78 This has a major impact on cardiac myocyte function i.
O-GlcNAcylation in the Liver Controls Systemic Energy Homeostasis
The liver typically performs these functions in response to hormonal signals from the pancreas (and to a lesser extent other organs). In response to rising glucose levels, the pancreas briefly secretes insulin, which promotes the uptake of glucose and glycogen and fatty acid synthesis in the liver (inversely, inhibits gluconeogenesis).
Roles for O-GlcNAc in Neuronal Function and Neurodegeneration
In 2013, it was demonstrated that diabetic hyperglycemia induced O-GlcNAcylation of CaMKII in the heart and brain, resulting in chronic activation of CaMKII-dependent pathways. The result of this is a fairly significant difference in the time between enzyme inhibition and treatment, which is likely to differentially engage known compensatory mechanisms.51,81,83 Moreover, the studies almost uniformly used different protocols to alter O-GlcNAcylation, assess LTP and LTD, and investigate mechanisms, further highlighting the need for more systematic studies of the role of O-GlcNAcylation in the regulation of synaptic plasticity.
Conclusion and Outlook
Moreover, because previous studies mostly focused on the role of O-GlcNAcylation in the regulation of individual cellular pathologies, e.g. This emerging topic of O-GlcNAcylation, which serves as a central regulator of neuronal function, homeostasis, and disease, is ripe for further investigation.
The O-GlcNAc transferase gene is located on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Cross-talk between two essential nutritional-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK).
Abstract
Introduction
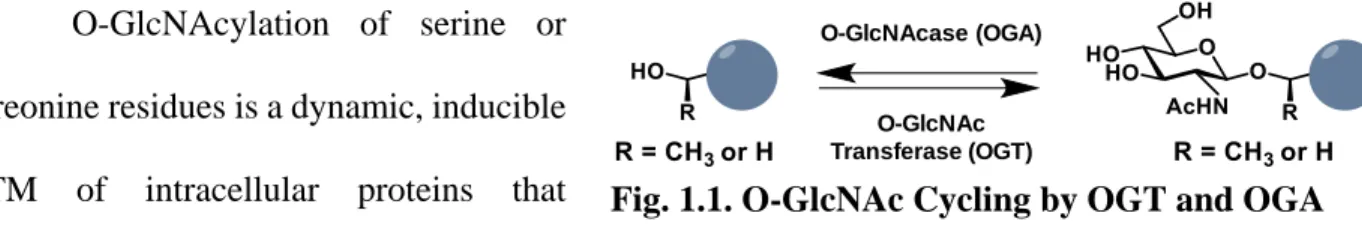
Herein, we describe a chemoenzymatic labeling approach that uses engineered bovine β-1,4-galactosyltransferase 1 (Y289L GalT) to label O-GlcNAsylated proteins with a GalNAz group (Fig. 2.1a). When combined with peptide:N-glycosidase F (PNGase F) treatment to remove GlcNAc-containing N-linked glycans30, this method allows for the specific, unbiased and global labeling of O-GlcNAsylated proteins.
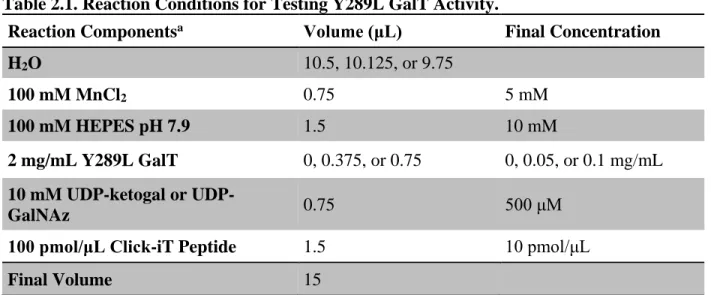
Expression and Purification of Y289L GalT
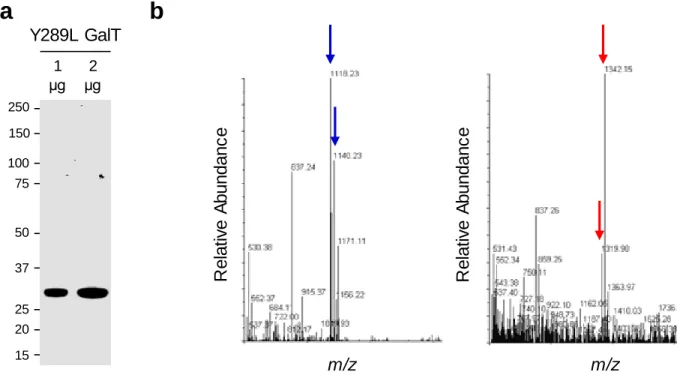
It is important to ensure that the pellet is completely resuspended at each washing step to ensure efficient purification of Y289L GalT. We usually obtain 5-10 ml of active Y289L GalT at a concentration of 2 mg/ml via the purification procedure described above.
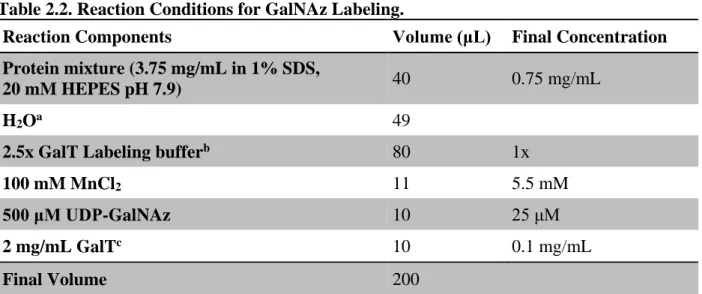
Labeling of O-GlcNAcylated Proteins with GalNAz
Although protein precipitation is usually not sufficient to significantly affect concentration, it is important to periodically recheck both protein concentration and activity to ensure consistent labeling in subsequent procedures. Measure the protein concentration of the sample using the BCA assay and dilute the sample to 1-2 mg/mL prior to reduction and alkylation.
CuAAC “Click” Reaction with Small Molecule Alkyne Probes
After labeling O-GlcNAcylated proteins from a cellular lysate with biotin or TAMRA, total levels of protein O-GlcNAcylation can be quantified. Alternatively, TAMRA-labeled proteins can be separated by SDS-PAGE and subjected to direct in-gel fluorescence analysis.
CuAAC “Click” Reaction with PEG Alkyne
Redissolve the protein in the desired volume of loading buffer for SDS-PAGE and Western blot analysis (see section 2.6.4 below). Lysates were separated by SDS-PAGE and analyzed by Western blotting using antibodies against the protein(s) of interest.
Biotin/TAMRA Immunoprecipitation
TAMRA
Resuspend the beads with 50 μL of 2% (w/v) hydrazine monohydrate and incubate with rotation at the end for 1 h at room temperature. Resuspend the beads in 50 μL of PBS and incubate with end-to-end rotation for 5 min at room temperature.
O-GlcNAc Site Identification
We believe that this new probe has significant potential to facilitate the identification of O-GlcNAc sites. Regardless, it is important to take into account the variable addition of the mass label (Daltons) to serine and threonine residues in the analysis.33 In summary, we believe that the outlined procedure can aid in the detection of low-abundance or otherwise difficult to identify O-GlcNAc sites.
Integrated Workflow for Discovery and Biological Assessment of Novel O-GlcNAcylated Proteins. Proteins
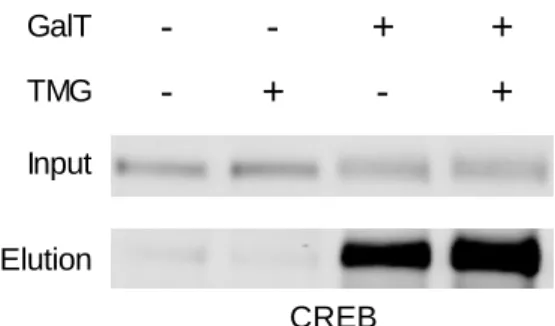
Again, we have found CREB to be an excellent positive control for the strategies described in this section. As an example, the increase in O-GlcNAcylation of CREB stimulated by TMG treatment can be seen in Fig.
Conclusions and Future Directions
The commercial availability of the reagents and the simplicity of the experimental approach should facilitate the adoption of this methodology by non-experts. Combining techniques with established methods such as stable isotope labeling with amino acids in cell culture (SILAC),53 isobaric labeling for relative and absolute quantitation (iTRAQ),54 or tandem mass labeling (TMT)55 should allow direct comparison of O -GlcNA they encounter different physiological states.
Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation by the ubiquitin ligase NEDD4-1. Optimization of chemoenzymatic mass labeling by strain-promoted cycloaddition (SPAAC) for determination of O-GlcNAc stoichiometry by Western blotting.
Abstract
Introduction
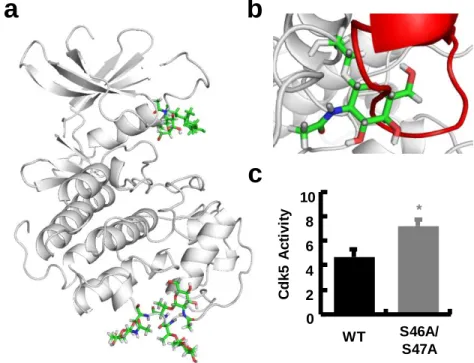
Specifically in AD, we believe that the following general conclusions are warranted: (a) increases in Cdk5 activity trigger a wide range of neurodegenerative events along with profound synaptic dysfunction and (b) pharmacological or other inhibition of Cdk5 has beneficial effects in models of neurodegeneration. 32,52-54 Despite the strong body of literature implicating Cdk5 in AD and other neurodegenerative diseases, relatively little is known about how or why Cdk5 activity may increase in AD. Herein, we first confirm that Cdk5 activity is increased in association with OGT KO.
OGT KO Increases Cdk5 Activity and p25/35 Binding
Together, this suggests a reduction in Cdk5 expression or protein stability that may be a compensatory response to increased Cdk5 activity. Interestingly, we also found that OGT IPs with Cdk5 confirm a direct interaction between the two proteins and suggest that Fig. OGT KO Increases Cdk5 Activity and Affinity for p35. a) Lentiviral transduction of floxed-OGT neurons with Cre results in efficient knockout of OGT versus an mCherry control.
Cdk5 is Dynamically O-GlcNAcylated in Neurons
These cells were also co-transfected with OGT and treated with TMG and KCl to maximally increase Cdk5 O-GlcNAsylation stoichiometry and thus our chances of identifying O-GlcNAsylated peptides. During the course of these experiments, Ke and co-workers also identified it and a Fig. Neuronal Depolarization Dynamically Induces Cdk5 O-GlcNAcylation.
Mutation of Potential Cdk5 O-GlcNAcylation Sites Increases Cdk5 Activity in N2A Cells. Cells
Excitingly, we could detect expression of all 4 constructs (Fig. 3.4b) at approximately the same level as endogenous Cdk5 (Fig. 3.4c). In future experiments, we hope to use this lentiviral KD and replacement system to confirm whether O-GlcNAc-deficient Cdk5 has increased activity in the Fig. Lentiviral Transduction of Neurons with. shRNA.
Conclusion
Experimental Methods
The supernatant was then removed and the amount of ADP was estimated with ADP-Glo according to the manufacture's protocol. The remaining volume was then incubated with streptavidin magnetic beads (ThermoFisher) for 1.5 h in the dark.
Inducible Knockout of p35 Cyclin-Dependent Activator Kinase 5 Alters Hippocampal Spatial Coding and Neuronal Excitability. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration.
Abstract
Introduction
Herein, we will highlight state-of-the-art technologies for identifying O-GlcNAc sites and tracking their context-specific dynamics. We will then describe emerging technologies for quantitative, MS-based O-GlcNAcomics and discuss how such approaches provide new, systems-level insights into the site-specific dynamics of O-GlcNAc in response to different cellular or disease states.
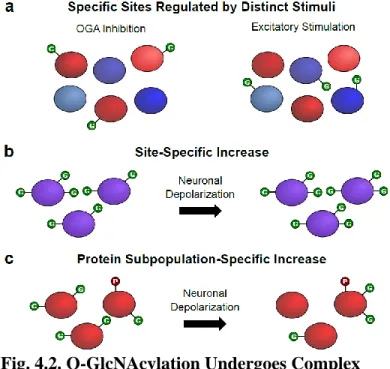
O-GlcNAc Site Identification on Individual Proteins
Quantitative release of O-GlcNAcylated peptides is then achieved using hydroxylamine or hydrazine, which imparts an additional positive charge to the peptide and thus facilitates LC-MS/MS analysis. O-GlcNAc sites on proteins, which are difficult to detect or are scarce, require techniques for highly efficient enrichment and release of O-GlcNAcylated peptides.
O-GlcNAc Site Mapping Across the Proteome
However, at the site level, the number of O-GlcNAc sites that were also phosphorylation sites did not exceed what would be expected by chance, and there was no tendency for O-GlcNAc and phosphorylation sites to cluster in primary sequence or three-dimensionally. space. Collectively, studies of the O-GlcNAcylated proteome have provided critical insights into the physiological functions of O-GlcNAc in the brain.
Methods for Measuring Dynamic O-GlcNAc Stoichiometry
As described below, knowledge of the site occupancy and inducibility of specific sites can provide insight into the importance of specific glycosylation events and the cellular contexts in which they function. strategy described above in combination with immunoblotting to detect the protein of interest. Importantly, the PEG tag shifts the molecular weight of the glycosylated species and allows them to be resolved from the non-glycosylated species by SDS-PAGE after immunoblotting to detect Fig. Chemoenzymatic labeling with PEG tags to determine O-GlcNAcylation.
Quantitative O-GlcNAcomics
The labeled peptides are then enriched, eluted from the beads by UV cleavage and subjected to LC-MS/MS analysis. Labeled proteins are then enriched, digested, eluted from the beads and analyzed by LC-MS/MS.
Conclusions and Future Directions
In the future, the integration of quantitative O-GlcNAcomics data with other modern "-omics" techniques (e.g. interactomics, phosphoproteomics, transcriptomics, proteomics) will also help to reveal important correlations in biological systems and the interplay between O-GlcNAcylation and other signaling networks. Together, we believe that multimodal, systems-level approaches coupled with rapidly developing technologies for quantitative O-GlcNAcomics will usher in a new era in our understanding of the functional roles played by O-GlcNAc.
Site-selective detection and analysis of O-GlcNAc-modified glycopeptides by coupled β-Elimination and electrospray mass spectrometry. Mapping O-GlcNAc modification sites using affinity tags for serine and threonine posttranslational modifications.
Abstract
Introduction
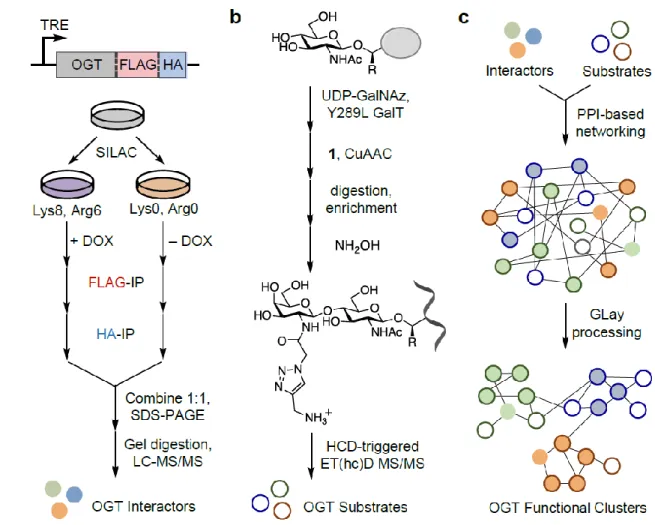
Although critical advances, these studies do not encompass the full breadth of the changing role that O-GlcNAcylation plays in these contexts. Together, this workflow produces a holistic, proteome-wide view of OGT interactions and substrates for a given biological condition.
Identification of OGT-Interacting Proteins
The attached FLAG and HA tags were then used to sequentially immunoprecipitate OGT and its associated proteins (Fig. 5.3a). Notably, our method confirms stable interaction between OGT and retinoblastoma binding protein 5 (RBBP5), nuclear pore glycoprotein p62 (NUP62), WD repeat-containing protein 5 (WDR5) and HCFC1 (Fig. 5.3b).