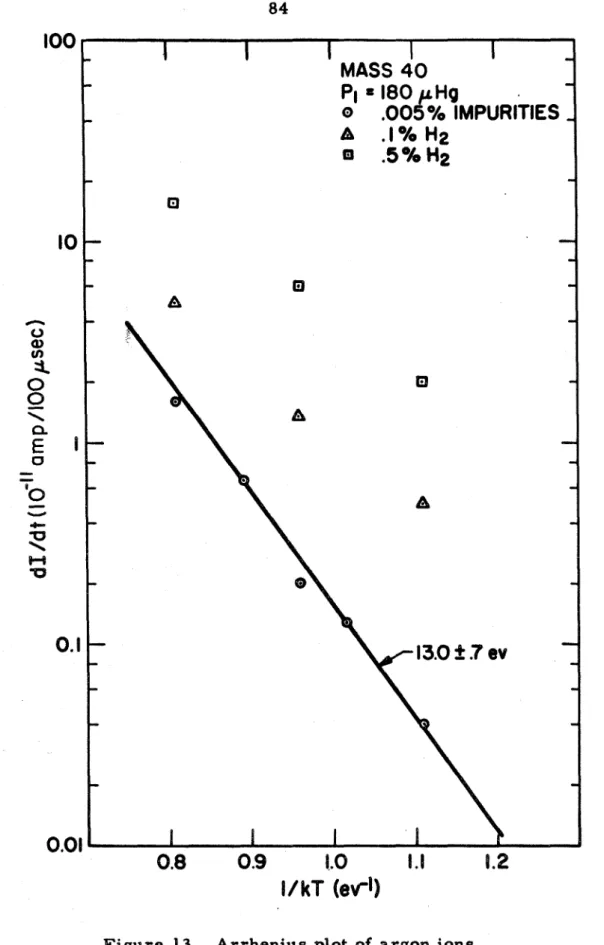
A detailed analysis of ion diffusion through the end wall of the boundary layer to investigate the effect of the sampling p r o c e s s on the reaction and response produces a r e described. Therefore, the relaxation time (or ionization time) is determined by the ionization kinetics of the initial stage of the. They found that the effective activation energy for ionizing argon coincides within experimental e r r o r with the excitation energy of the f i r s t electronic excited S t a t e r a t h e r as with the ionization energy, indicating that the initial ionization stage proceeds with two-step p r o c e s s s a s proposed by Weymann, but that the excitation step is r a t e controlled.
However, a simple analysis is made with some basic assumptions to demonstrate the essential metrics of the sampling p r o c e s s . A description of the inelastic collision process, the r a t equations, and various models of the ionization process is given in the next section. Then the total energy E of the system i is equal to the center of mass energy Ec plus the relative energy E That i s.
Fortunately, our main interest, the reaction rate, depends on the integral of the cross section weighted by the Boltzman factor. It depends on the energy states of the electron of each atom, the interaction potentials between the atoms, the relative energies of the particles, Therefore, during transient phenomena in which the population of the excited state increases to i t s v t steadystate1 value, + increases with.
F u r t h e r m o r e , assuming the effective lifetime of excited intermediate equations with r e s p ec t to radiative decay and collisional de-excitation sufficiently long (s is assumed in the two-step equations).

SAMPLING PROCESS
In the ca s e itself, there is always a layer of cool and dense gas (thermal la y e r) next to the end wall. Knudsen number Kn = h*ld where A * i is the mean path to the sonic point in the sampling orifice, d is the diameter of the sampling orifice. The problem arose here is to find the charged particle density distribution in the thermal layer where the gas density is so high that the local mean is much smaller than the orifice diameter.
A continuum description that includes the effects of viscosity, heat conduction, diffusion of the many species, and elec. As pointed out by Su (11965), quasi-neutrality is a good approximation for ion and electron diffusion in the thermal layer up to the edge of the sheath layer, which is about a few Debye lengths from the end wall. The u p s t r e a m influences of the jet on ion diffusion in the d e r m a l layer and the downward s t r e a m interaction of the jet with the acceleration.
At continuity, the up s t r e a m velocity induced by the jet is proportional to the i n v e r s e s of the number density and the i n v e r s e of the distance squared. The downstream interaction of the beam with the electrodes is extremely complicated and almost impossible to analyze. Due to the strong attractive field and geometry, most of the ions are collected by the extraction electrode (Figure 4); only 0.
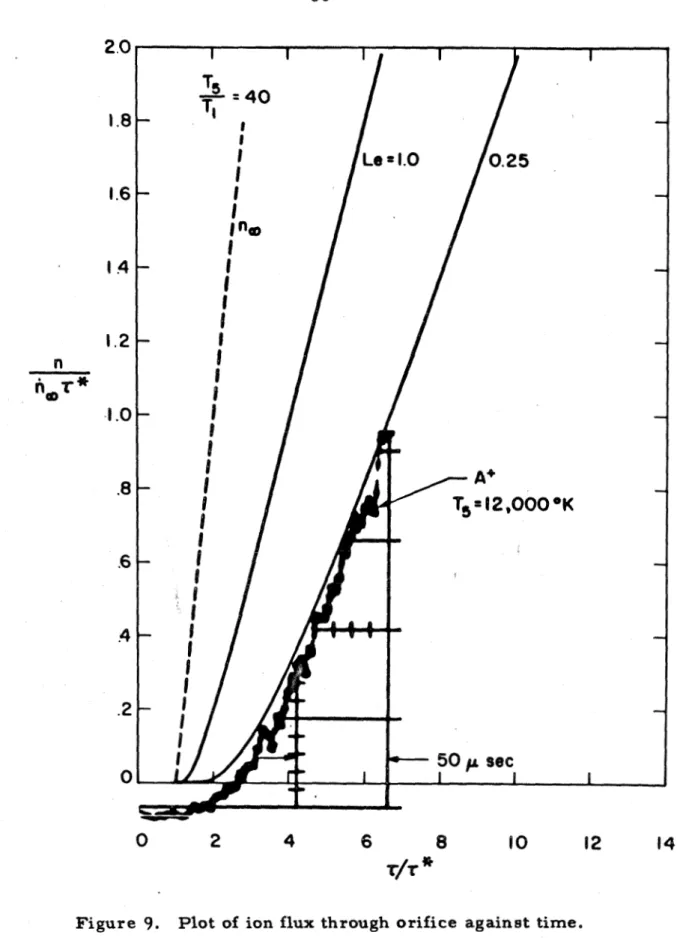
The upper l i m i t is due to the fact that both the effect of the electrostatic sheath in front of the extraction electrode and the effect of the mutual repulsion of the ions in the beam become important. The agreement with the actual mass spectrometer output of argon ions further demonstrates that the sampling technique can be used for accurate measurements of the ionization processes. It was assumed in the previous section that reactions in the thermal layer are frozen or negligible.
It is noted that although this charge does not result in a number of ions if they diffuse through a neutral gas of the same species (i.e., it is not a chemical change), it is nevertheless a diffuse 1. ion coefficient. This exponential form is due to the character of the collision, the ions approaching the atoms with a velocity very small compared to that of the atomic electrons. On the b a s i s of the above r e s u l t s i t , it can be concluded that in these experiments the reactions in the e r m a l a r have a negligible effect on the diffusion p r o c e s , provided the amount of impurity is not too high (l.
This calibration constant increases in the same magnitude with the calculated value that takes into account the long-time diffusion (the ratio between the density of ions in the shock-heated gas and the density in the aperture. Using this calibration constant, it is possible to obtain the absolute values of the hydrogen cross section in the ionization collision. It is possible that is the calibration constant dependent on mass, or that the rate constant obtained by Kelly is too high (more comparison with Kelly's results, Bate r , will be given).
The test conditions (Table B and Appendix A) in this experiment are chosen so that the test time is long, the interaction v. The ionization rate of the hydrogen atom is fast and a quasi-equilibrium or steady state is reached, meaning that the hydrogen is in equilibrium with the argon, which is still at freezing temperature and slowly ionizing. The oxygen ion traces behave more or less like hydrogen, but at the end of the test time the ion current still approaches the equilibrium level.
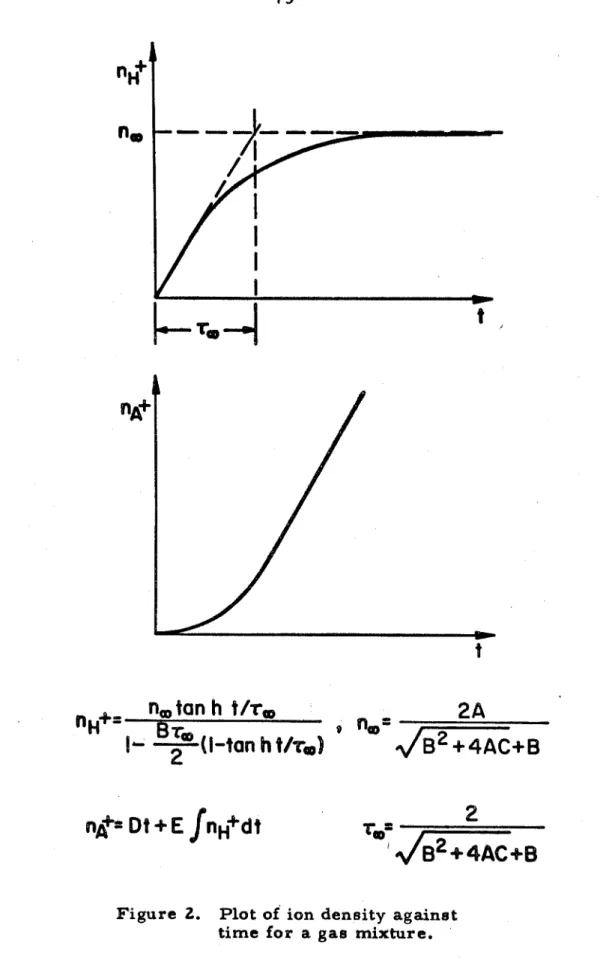
Within the limit of experimental accuracy, the characteristic of the t r a c e s indicates that the initial stage of ionization does a g r e with the multi-step ionization model, (where radiative decay and the collisional de-excitation are sufficiently slow compared to the ) and the theoretical calculation of ionization in a gas rnixt.cn r e. The simplified theoretical model of ionization in a gas mixture cannot possibly do more to describe these complex processes than to provide a crude demonstration of the enormous observed rates of hydrogen ionization. The relative size of the effective k r o s s section SHA (section 2. 1, equation 12) can be determined by ratio.
HA can be obtained by using the calibration constant of the m a s s spectrometer, the m e a s u r e d activation energy and the measured hydrogen ion c u r e n t . It is linear with time and never becomes saturated before the end of t e t time, unlike hydrogen ion t r a c e s . It can be argued from the r a t e equations (section 2. 3) and the Saha equations (Appendix A) that the Pack sf saturation in the xenon t r a c e s is due to a low activation energy and the saturation of the hydrogen t r a c e due to a high exponential factor (as, in fact, i t i s .
The ion density around the plateau region is a r f r o m the equilibrium ion density; only about B p e r percent of the equilibrium value. This is believed to be due to the formation of a sheath in front of the withdrawable electrode and the effect of charge reflection in the ion beam. The result shows that the initial ionization of xenon is due to atom-atom ionization with activation energy E = 8.3 ev and an.
CONCLUSION
As the gases behind the reflected shock wave begin to ionize, equilibrium conditions will deteriorate beyond a certain ionization.
The metastable state of an atom is the excited state which, due to the J-selection rules, cannot jump to a lower energy t a t e by electric dipole radiation. Note that i s a l s o can decay to a lower energy state by electric-quadrupole or magnetic-dipole radiation with an inherently long lifetime (Phelps 1953). For most gases, the lifetime of the excited state (decay by electric-dipole transition) is of the order of 10 -8 sec.
FOR the metastable state, the lifetime depends on the energy difference between levels and the wave functions. The spontaneous transitions from the 2s state to the 2P state have a negligible s m a l l probability, due to the s m a l l energy difference. The magnetic-dipole transitions from 2S to 1 s are not strictly forbidden, but their probability is smaller than that for allowed electric-dipole transitions by a factor of 10 -13.
The average lifetime due to this transition was calculated as orphan by Breit and Teller (1940). The effect of an electric field on the lifetime of the metastable state in hydrogen is discussed by Bethe and Salpeter (1957). Due to the electric field the 2S state is mixed with the 2P state giving an average lifetime of.

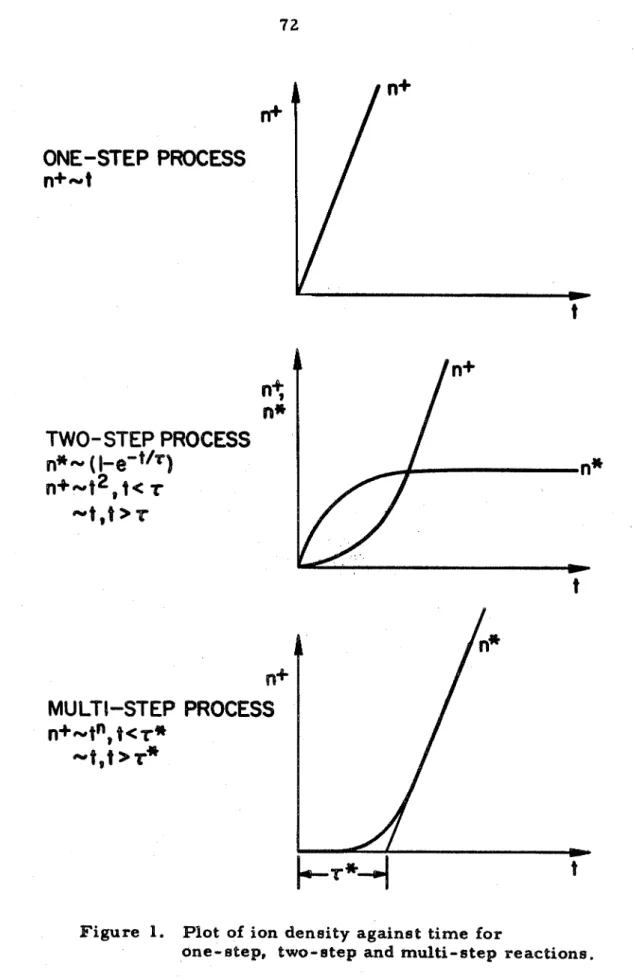
ONE-STEP
TWO- STEP PROCESS
PROCESS
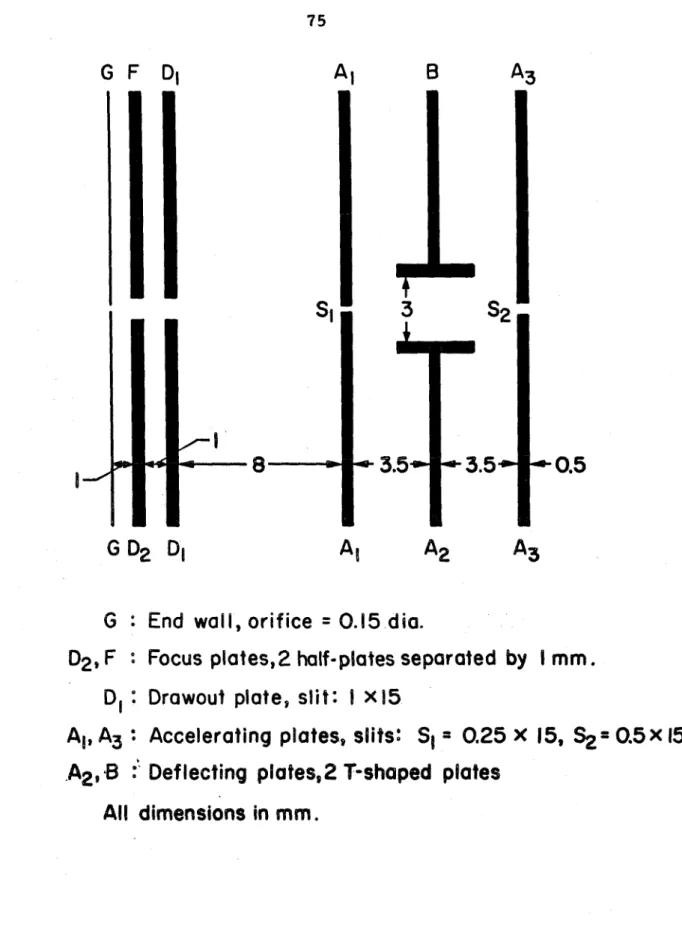
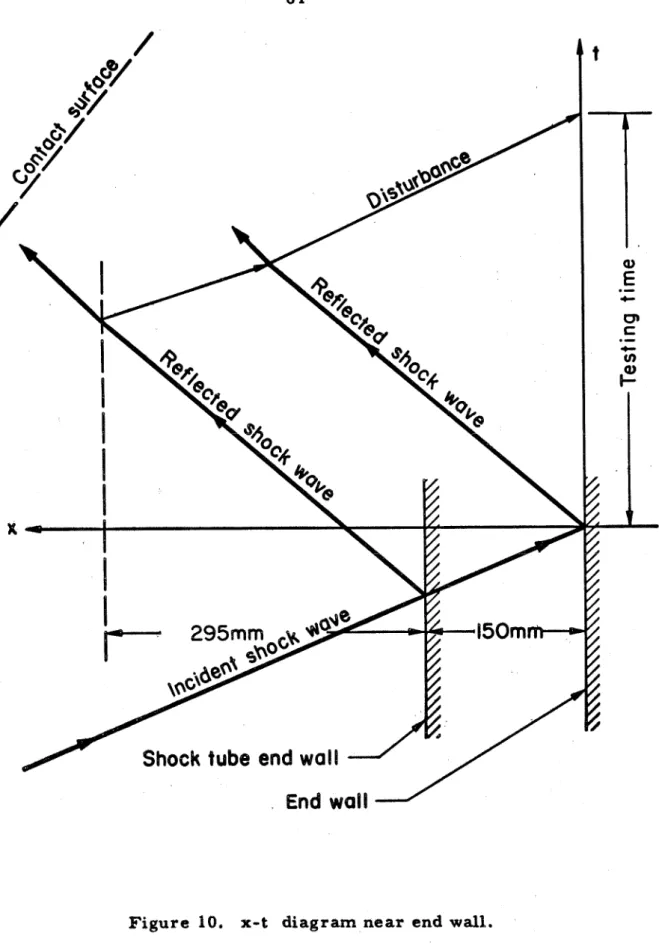
All dimensions in mm,
Shock tube end wall