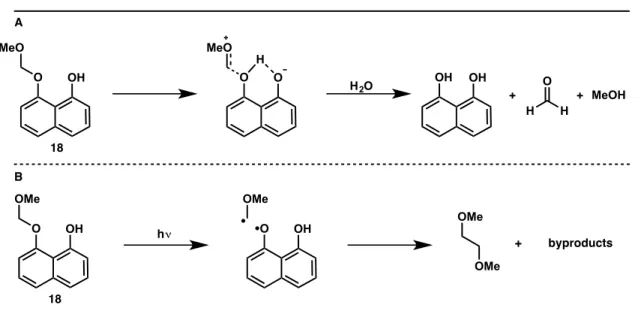
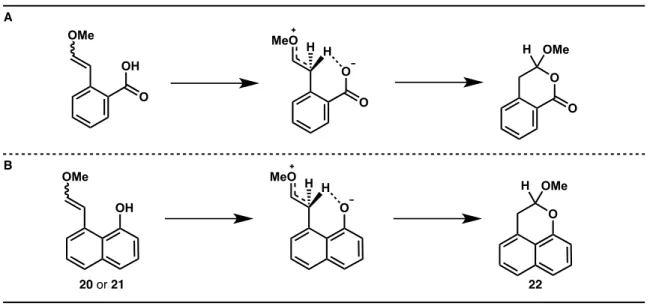
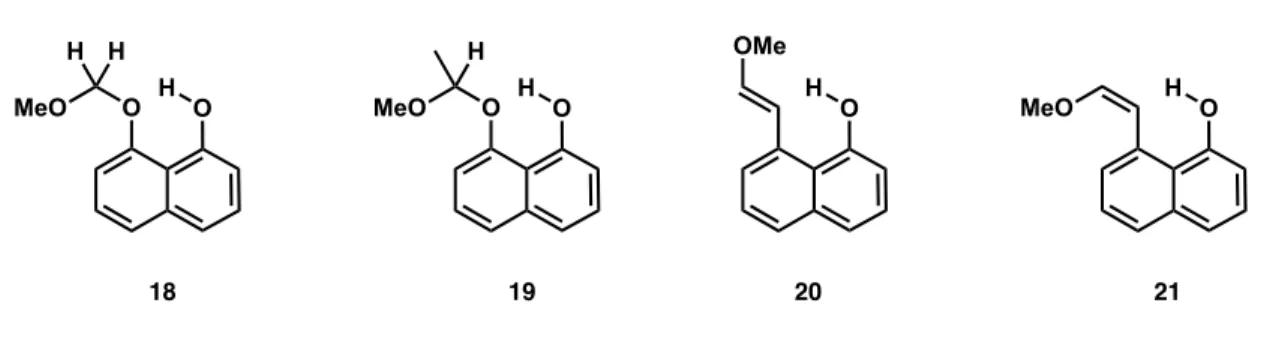
I am very grateful to have been involved in the development of the benzoquinone trimethyl key photochemistry. Initial model systems are designed to address this challenge using the photoacid effect.
INTRODUCTION
This is mainly due to the complexity and diversity of the biochemical pathways involved in the secondary injury. Excitotoxicity is caused by upregulation of the neuronal excitatory pathways, mainly mediated by the neurotransmitter glutamate.
THE UTILITY OF EXCITED-STATE PROTON TRANSFER IN THE DEVELOPMENT OF NOVEL PHOTOCHEMICAL REACTIONS
INTRODUCTION
A major challenge in developing light-activated drug delivery strategies that absorb in the near-infrared is the very small energy associated with these wavelengths of light. The research presented here focuses on certain advances made in the development of novel photoacids-based drug delivery systems.
T-BUTYL ESTER MODEL SYSTEMS
The formation of indolenine 8 was also challenging due to the instability of the electron-rich aryl hydrazine (Scheme 3). The initial proton transfer from the excited naphthol to the t-butyl ester results in the formation of a zwitterion, which then undergoes cleavage of the C-O bond of the t-butyl ester in a second step.
GENERAL-ACID CATALYZED SYSTEMS
For an acid in the excited state (*HA), the process catalyzed by a specific acid competes directly with the deactivation of the excited state (𝑘J), as shown in Scheme 6. However, the rate constant 𝑘J is only present in the photochemical process, and represents the intrinsic lifetime of the excited state.
BIMOLECULAR SYSTEMS
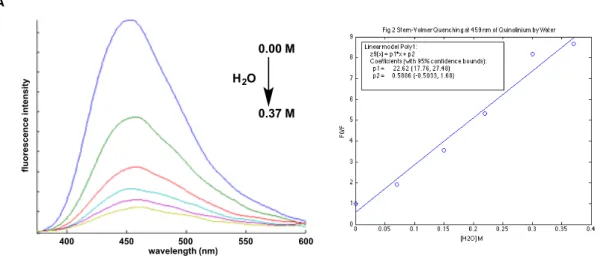
The Stern-Volmer quenching constant, 𝐾, W, allows the quantum yield for quenching (ΦZ) to be calculated at a given quencher concentration ([Q]) according to Eq. For a photochemical reaction that is initiated by proton transfer to the excited state, ΦZ represents the maximum efficiency for this step. However, no substrates were identified that led to appreciable quenching of naphthol fluorescence, suggesting that efficient excited-state proton transfer reactions cannot be achieved in a bimolecular manner for these short-lived and weak photoacids.
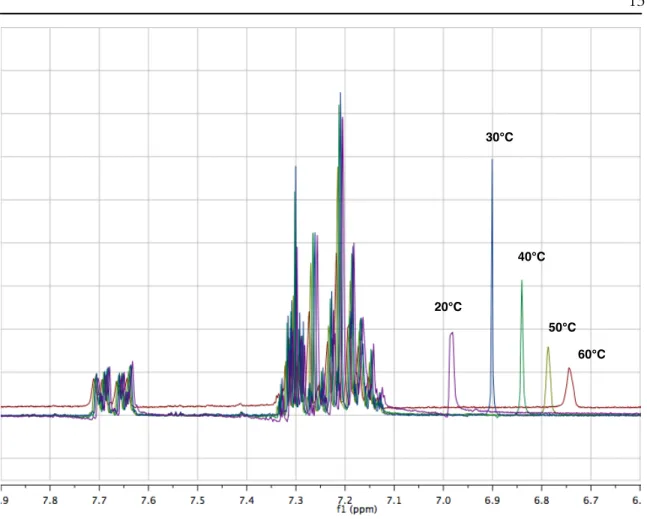
A Stern-Volmer constant, 𝐾,W, of 22 M-1 is obtained by linear regression analysis of the maximum fluorescence intensity (Figure 10A). the hydroxyindolinium 25 shows a modest fluorescence enhancement with a 𝐾,W value of -0.6 M-1 after addition of water in acetonitrile. These results support previous findings that 24 is an excited-state acid capable of protonating water, while 25 is unable to do so, either because it is not an excited-state acid or because the excited-state duration is too short to undergo efficient bimolecular processes. proton transfer at these concentrations of quencher. If this product were the result of a proton transfer process in the excited state, a secondary oxidation step would have to be invoked.
CONCLUSIONS
EXPERIMENTAL
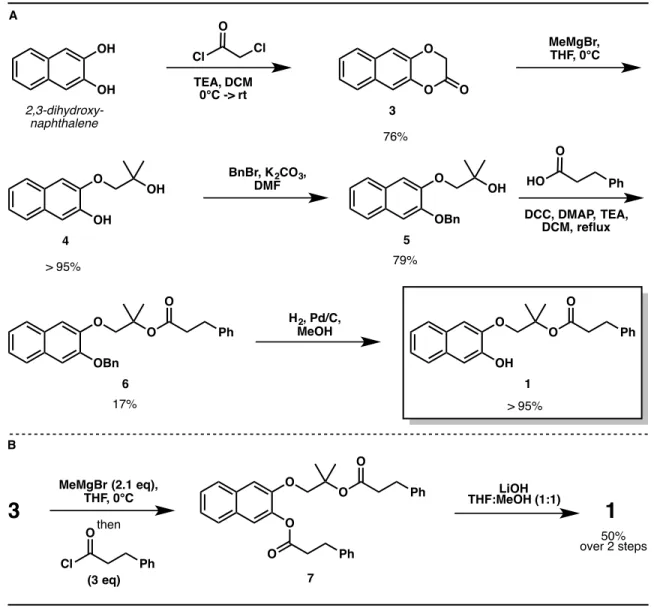
The crude material was purified by flash column chromatography (SiO2, 25% EtOAc in hexanes) to afford 5 as a clear oil. The crude material was purified by flash column chromatography (SiO 2 , 5-10% EtOAc in hexanes) to give 6 as a clear oil. The crude material was purified by flash column chromatography (SiO 2 , 25% EtOAc in hexanes) to afford 1 as a clear oil.
The residue is purified by flash column chromatography (SiO2 .75% EtOAc in hexanes) to afford 8 as a clear oil. Freshly distilled tBuOH (3 eq) is added dropwise and the reaction is stirred at room temperature for 30 minutes. The crude oil is purified by flash column chromatography (SiO2, 10% EtOAc in hexanes) to afford 22 as a clear oil.
Excited-state proton transfer: From confined systems to 'super' photoacids to superfast proton transfer \dag. The position of the six-membered intramolecular hydrogen bond as a switch for the promotion of an excited-state intramolecular proton transfer (ESIPT) in O-hydroxynaphthoic acid esters. Intramolecular catalysis of the hydrolysis of an acetal by an internally hydrogen-bonded hydroxy group.
Efficient Intramolecular General Acid Catalysis of Vinylether Hydrolysis by a Neighboring Carboxylic Acid Group.
MECHANISTIC STUDIES ON THE TRIMETHYL LOCK CYCLIZATION OF SULFUR-SUBSTITUTED BENZOQUINONES TRIGGERED BY VISIBLE LIGHT
INTRODUCTION & SYNTHESIS
MECHANISM INVESTIGATIONS ON THE TRIMETHYL LOCK CYCLISATION OF SULFUR SUBSTITUTED BENZOQUINONES INDUCED BY VISIBLE LIGHT. Of course, deprotection of the phenol can be performed photochemically using established cage groups5,6, but this approach does not naturally lead to longer wavelength systems. Although most quinone photoreductions involve amines15–22, we chose a sulfide as the potential electron donor in our original design (Figure 1C).
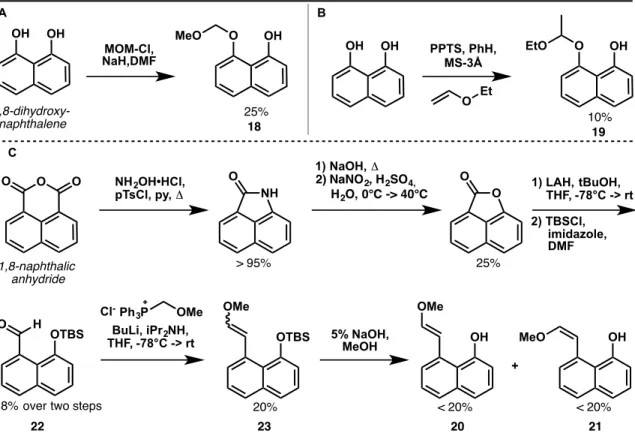
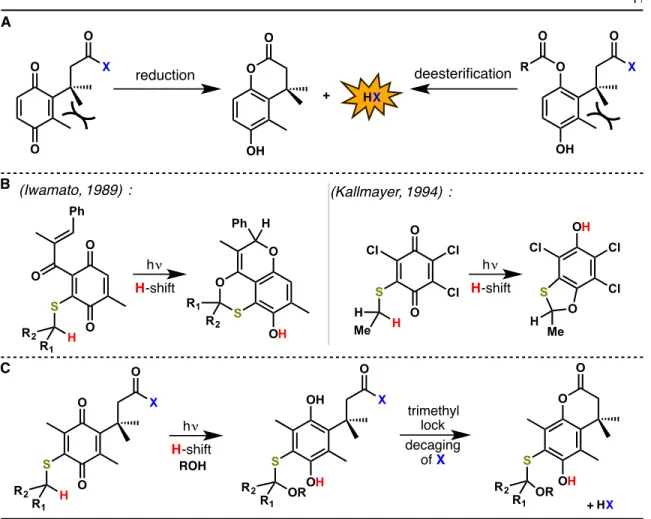
We expected easier synthesis of the desired systems, more favorable redox properties, and perhaps greater stability in air and in a biological context. The synthesis (Figure 2A) is efficient and allows a wide variety of sulfide substituents to be introduced in the final step (Figure 2B). Note that the last two steps in the synthesis can also be reversed one after the other.
STEADY-STATE PHOTOLYSIS
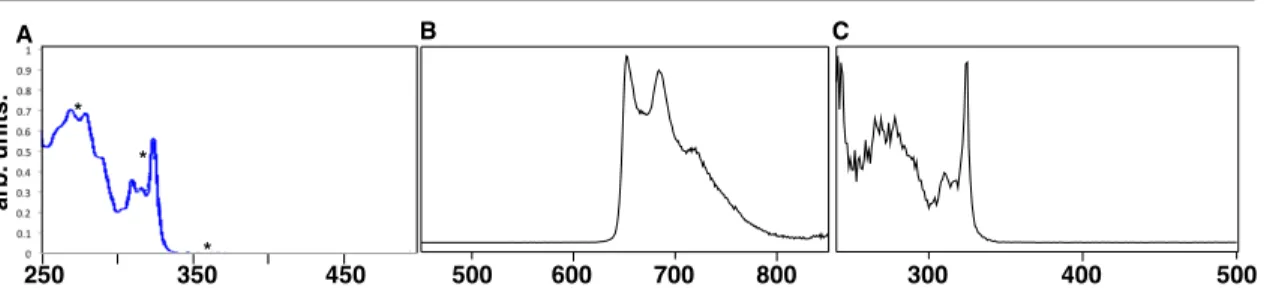
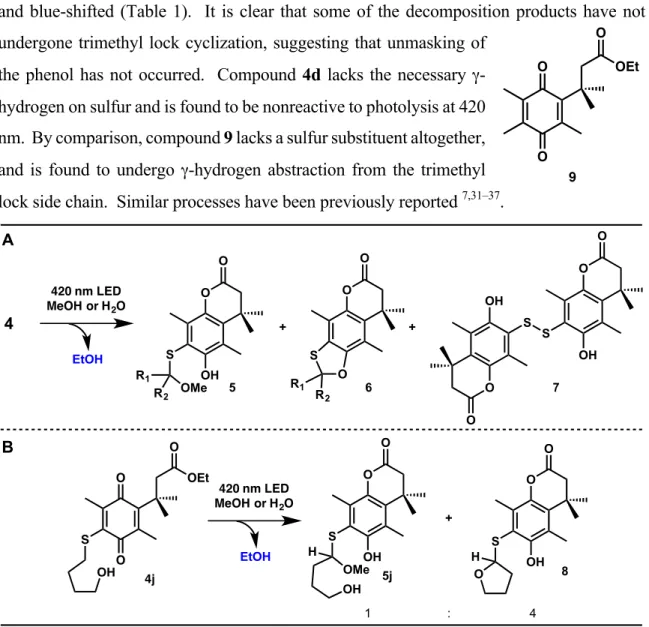
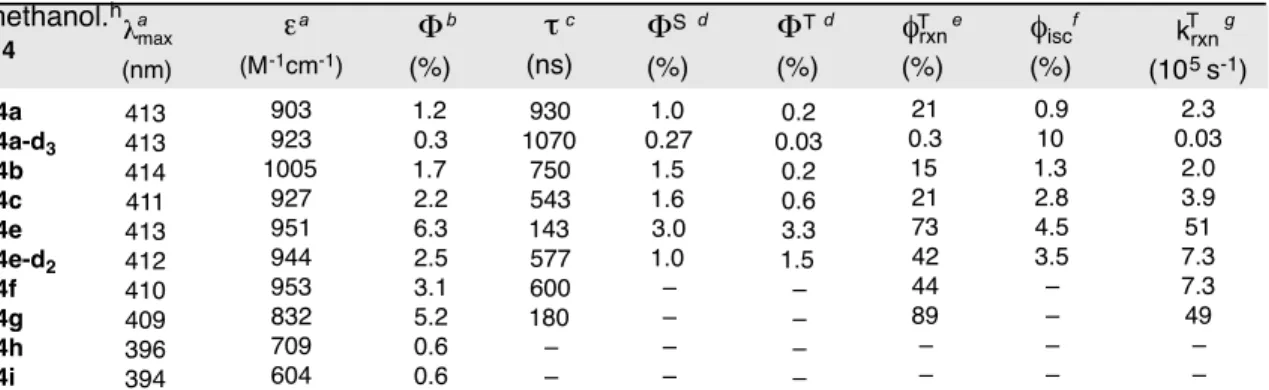
Notably, a broad visible absorption band is observed at approximately 413 nm; the relevant data for this band (λmax and ε) were collected for key substrates and are reported in Table 1. We could not observe luminescence from 4a either in liquid media at room temperature or at 77K in a frozen matrix, as is typical of quinones23–30. Along with the expected methanol adduct 5, in some cases the cyclic thioacetal 6 is also produced, presumably by intramolecular trapping of the species trapped by methanol or water.
It is clear that some of the degradation products did not undergo cyclization of the trimethyl lock, indicating disclosure. Compound 4d lacks the necessary γ-hydrogen on the sulfur and is found to be unresponsive to photolysis at 420 nm. In comparison, compound 9 lacks the sulfur substituent entirely and was found to undergo γ-hydrogen abstraction from the trimethyl side chain.
MECHANISTIC INVESTIGATIONS
We have undertaken several studies to investigate the role and nature of the triplet state in the photoreaction. Stern-Volmer plot for the quenching of the quantum yield for disappearance of 4a (white diamonds) and 4e (black squares) by diethylaniline. According to this model, the quantum yield of the singlet (ΦS) due to direct photolysis of the quinone is defined by the path 𝑆3Zà 𝑆&Z à 11 à 10 à 5.
6 is denoted as ΦhBepgq (equation 7), and describes the sensitized quantum yield for quinone disappearance in the limit where deactivation of the triplet sensitizer occurs solely through collisions with the quinone. 8 for a low efficiency reaction, the lifetime ratio (𝜏)/𝜏|) approaches unity, and the KIE is mainly determined by the quantum yield ratio (𝜙)/𝜙|). Therefore, the accuracy in the KIE recorded for the 4a/4a-d3 system is mainly the result of an accurate measurement of the sensitized quantum yields (ϕcde).
MECHANISTIC INTERPRETATION
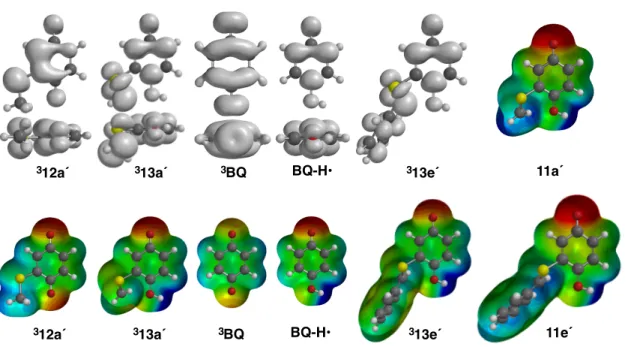
Together, these calculations strongly support the assignment of the triplet in Figure 9 as the charge-transfer structure, 312. 312 à 313 involves protonation of the semiquinone anion n-orbital with accompanying electron transfer to the sulfur radical cation. Also shown in Figure 10 is the electrostatic potential surface for the ground state of the zwitterion product, 11a´.
For the α-thioradical (313e´), significant spin density can be observed at the ortho and para positions of the aromatic ring. The main difference between 11 and 313 when considering the electrostatics of the coordinated (312 à 11) and stepwise (312 à 313 à 11) routes is the difference in the amount of positive charge on the substituent. Once completed, 100 µl of the photolysate is transferred to a 2 ml volumetric flask (the syringe is initially rinsed three times with a small volume of photolysate).
Using a 1 mL gas-tight syringe equipped with a 4-inch needle, 500 µL of the solution is transferred to the bottom of an 8-inch NMR tube, thawed three times with a pump, and then flame-sealed under vacuum. The latter is given by the absorbance of the Fe2+- phenanthroline complex measured at 510 nm (see general procedure above), the known extinction coefficient (11100 M-1cm-1) and the volume of photolysis.
DECAGING STRATEGIES BASED ON THE PHOTOREDOX CHEMISTRY OF METHYLENE BLUE
PHOTOOXIDATION OF TERTIARY AMINES
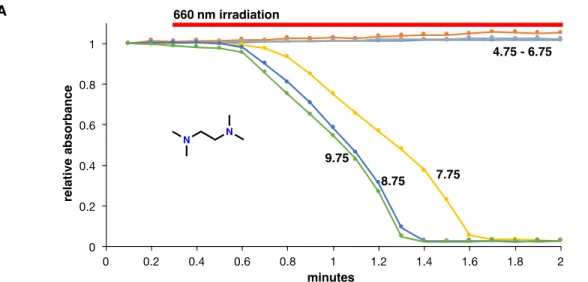
To initially probe the possibility of a bimolecular photoredox reaction between different amines and methylene blue, a 10 µM dye solution was photolyzed in the presence of 20 mM amine in water buffered at physiological pH (Figure 4A). The mixture of products resulting from the photolysis of methylene blue in the presence of various diamines is complicated by selectivity and oxygen sensitivity problems. A proposed model to describe the experimental observations for the photoreaction of methylene blue with diamines in buffered water at pH = 7.4.
Because this photochemical method is intended to be used in a biological environment, the sensitivity of the above-mentioned diamine oxidation processes to the presence of oxygen and the ability of methylene blue to sensitize singlet oxygen formation will need to be suppressed. Photolysis of 2 in buffered water in the presence of air results in a clean conversion to a product exhibiting a mass spectrum consistent with the doubly demethylated species, 3 (Figure 7B). As shown in Figure 8A, when methylene blue is photolyzed in air-equilibrated methanol, the intense absorption of DPBF at 400 nm decays rapidly due to singlet oxygen capture, which is known to result in the formation of an endoperoxide.
DECAGING VIA LACTAM FORMATION
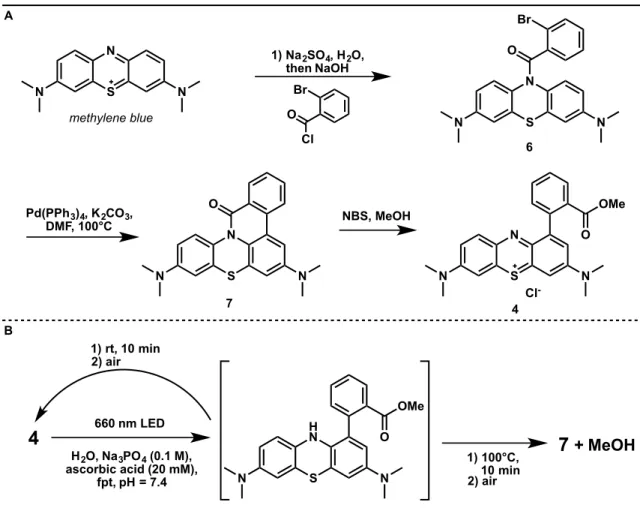
After the initial methyl group is hydrolyzed, the resulting leuco dye can be reoxidized by oxygen and re-enter the cycle to oxidize and hydrolyze the second methyl group. After reduction of methylene blue with sodium dithionite, the leuco dye can be blocked with 2-bromobenzoyl chloride to generate benzamide 6. These results suggest that cyclization of the reduced form of the dye is not efficient in the chamber.
A system analogous to 5 containing a substituted nitrobenzene had previously been reported to undergo rapid lactam formation upon chemical reduction of the nitro group to an aniline 34 . The photolysis of this compound in the presence of ascorbate rapidly results in the formation of 11 and decomposition of methanol at room temperature, even when carried out in air-equilibrated solvent (Figure 11B). A certain advantage of this system is that ring formation occurs with photobleaching of the chromophore, which prevents secondary photolysis.
CONCLUSIONS
EXPERIMENTAL
After stirring for a further 30 minutes, ortho-bromobenzoyl chloride (6.3 eq) is added dropwise, and the mixture is stirred for 12 hours. The crude material is purified by flash column chromatography (SiO2, 1% MeOH in DCM) to yield 7 as a yellow oil. After cooling with an ice-water bath, methacryloyl chloride (1 eq) is added dropwise and the solution is allowed to warm to room temperature and then refluxed overnight.
After stirring vigorously overnight, the mixture is extracted with DCM (100 mL x 3) and the combined organics are dried over MgSO4 and concentrated in vacuo. The solution is extracted with DCM (20 mL x 3) and the combined organics are dried over MgSO4 and concentrated in vacuo. A solution of NBS in methanol (1 equiv, 0.05 M) is added dropwise, and the reaction is stirred under an inert atmosphere for 15 min, at which point TLC (5% MeOH/DCM) indicates complete consumption of the starting material .
New trends in photobiology: Photochemical interactions of methylene blue and analogs with DNA and other biological substrates. Methylene blue restores spatial memory retention impaired by an inhibitor of cytochrome oxidase in rats. Oxygen uptake induced by electron transfer from donors to the triplet state of methylene blue and xanthene dyes in air-saturated aqueous solution.