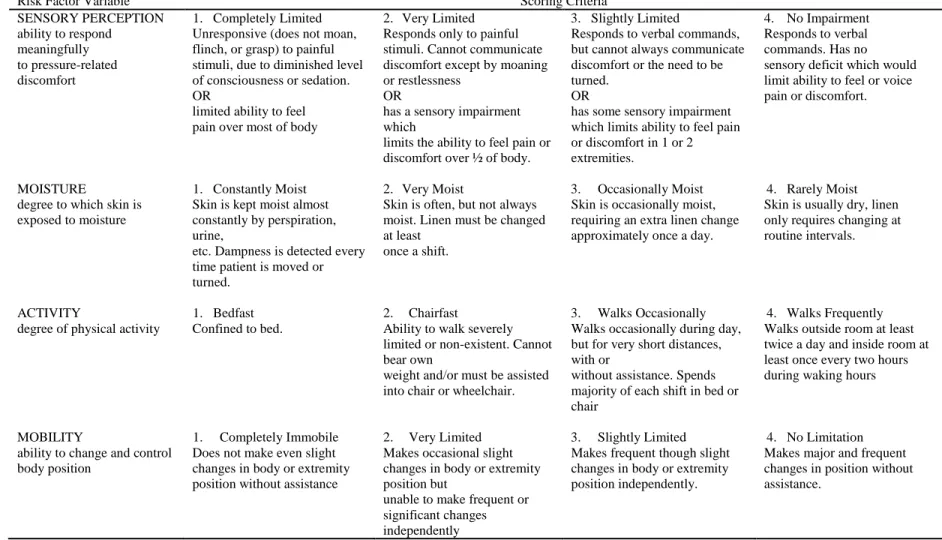
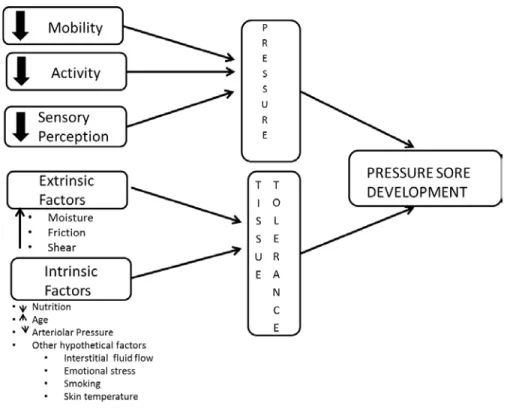
The literature identifies over 200 risk factors that may contribute to pressure ulcer development (Anthony, Parboteeah, Saleh, & Papanikolaou, 2008), but methodological and definitive inconsistencies complicate and slow the process of translating research into clinical practice (Keller, Wille , van Ramshorst , & van der Werken, 2002). According to Anthony et al. 2008), over 200 risk factors for pressure ulcer development have been identified in the literature. When measured both 48 and 24 hours before study enrollment, all Braden moisture subscale scores were statistically significantly associated with pressure ulceration.
Similarly, all Braden Friction/Shear subscale scores were statistically significantly associated with pressure ulcers when measured 24 hours before study enrollment. Summary of multivariate associations with pressure ulcers measured 24 hours before study enrollment for the full model. Summary of multivariate associations with pressure ulcers measured 48 hours before study enrollment for the full model.
None of the Braden subscale scores were statistically significantly associated with pressure ulceration in the final model using variables obtained at 48 hours to study enrollment. This finding is consistent with those reported in several studies of pressure ulcer risk factors. Critically ill patients are a vulnerable and understudied population with respect to pressure ulcer risk factors.
STATEMENT BY PERSON AGREEING TO BE IN THIS STUDY
Your decision to allow your family member/friend to participate in this research study is voluntary. If found fit, further participation in this study will only be possible with his/her consent. Please read this form carefully and ask any questions about this study.
If we learn anything new that may affect the risks or benefits of this study, you will be told so that you can decide whether you still want to participate in this study. The purpose of this study is to see if any risk factors you may have for developing a pressure ulcer are different from other patients who have developed a pressure ulcer. This includes treatments and tests you would need even if you were not in this study.
There are no side effects associated with this study, because the study does not involve additional or different treatment than you would normally receive due to your disease. The risks associated with this study are minimal and include possible loss of...anonymity because your medical record number will be linked to the information we...collect about you from the medical record. Because we only review your medical file, there is no chance that you will be harmed by participating in this study.
The medical record number will remain attached to the protected health information until the study is completed and the PI is satisfied that the data is complete and accurate. All my questions have been answered and I freely and voluntarily choose to participate in this study. A member of the study team will try to contact you in person or via
What will happen and how long will my family member/friend remain in the investigation. The treatment will be the same whether your family member/friend participates in this study or not. The overall findings of the study will be published in an appropriate journal after the findings.
Subject Information-CASE
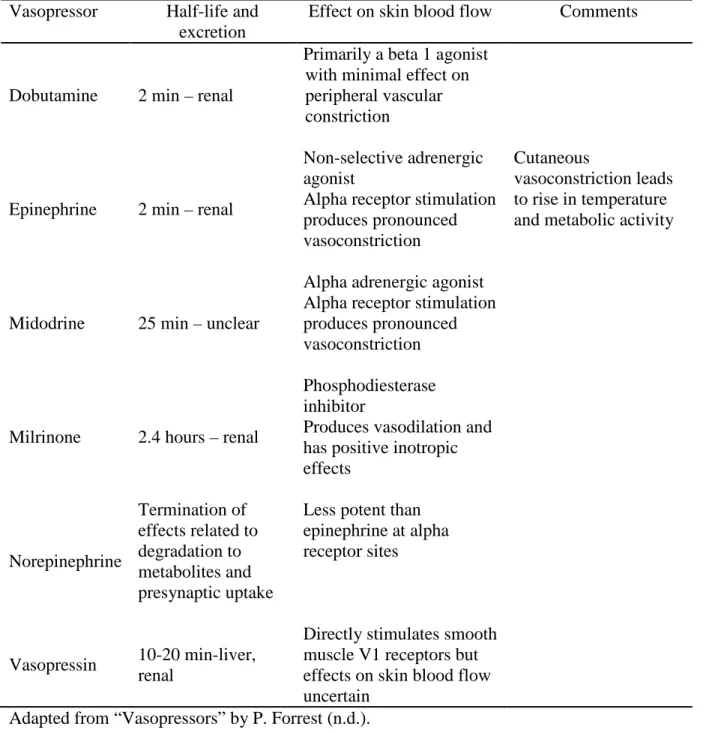
Does the patient currently need a ventilator? ). can be from Braden during the day or at night). can be from Braden during the day or at night). can be from Braden during the day or at night). can be from Braden during the day or at night). can be from Braden during the day or at night). can be Braden's during the day or at night. Total mcg/kg over a 24-hour block, 2 days before study enrollment (use midnight of study enrollment as reference). Total mcg/kg over a 24-hour block, 1 day before study enrollment (study midnight use).
In total units during the 24-hour block 2 days before study registration (use midnight of study . registration as reference)). Did the patient require pressor use at any point during the ICU stay BEFORE the 48 hour study. registration?. Is the patient currently in a hand brace or have hand braces been used within 48 hours of study enrollment (use midnight of study enrollment as reference)).
Subject Information-CONTROL
Data source for a prospective cohort study to determine pressure ulcer risk in critically ill patients. Objective: To determine the feasibility of using the electronic medical record (EMR) as a data source for a future study to identify risk factors for pressure ulcers in critically ill patients. Subjects: All patients 18 years of age admitted to the intensive care unit without a preexisting pressure ulcer during the study.
Data were abstracted from the EMR and entered into the database for pressure ulcer positive subjects. There was 100% agreement between staff nurse and expert on pressure ulcer status among pressure ulcer positive and negative subjects, and 83%. The predominant approach to investigating pressure ulcer risk factors in the related literature is the prospective cohort study.
Subjects in the study were randomly selected for reliability testing on the presence of pressure ulcers and Braden scores generated by the staff nurses. The results of the most recent Braden scores and pressure ulcer documentation were used and entered into the study database. Of the six subjects who developed a pressure ulcer during the 14 day study, there was 100% agreement between the staff nurse documentation and expert reviewer validation regarding the presence and location of the pressure ulcer.
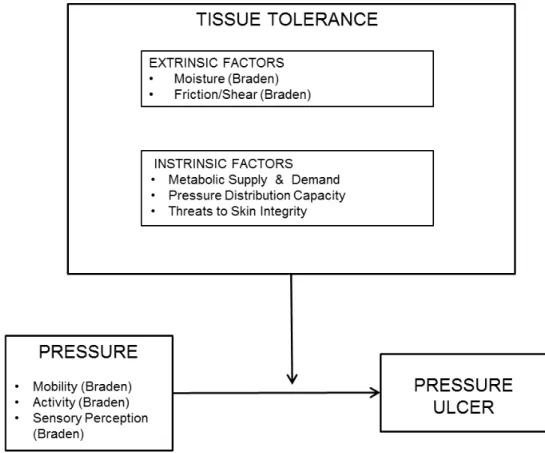
The agreement between the nurse and the expert assessor on the presence, location and stage of pressure ulcer-positive patients is encouraging. Risk factors for the development of pressure ulcers in critically ill patients: a conceptual model to guide research. Risk assessment of pressure ulcers in the intensive care unit: inter-rater reliability and validity studies of the Braden and Waterlow scales and subjective ratings in two intensive care units.
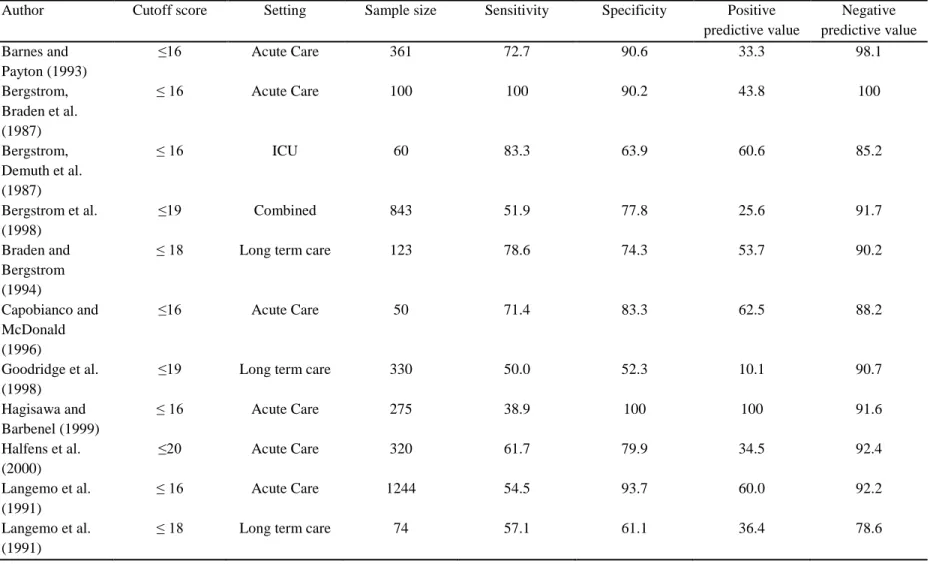
An interrater reliability study of the assessment of pressure ulcer risk using the Braden scale and the classification of pressure ulcers in home care. The Braden Pressure Ulcer Risk Scale: Evaluation of the Predictive Validity in Black and Latino/Hispanic Elderly. Predicting pressure ulcer risk: A multifactorial approach to risk factor assessment in a large university hospital population.