S M I T H S O N I A N C O N T R I B U T I O N S T O T H E M A R I N E S C I E N C E S • N U M B E R 21
Seagrasses from the Philippines
Ernani G. Mefiez, Ronald C. Phillips, and Hilconida P. Calumpong
ISSUED
DEC 11983
SMITHSONIAN PUBLICATIONS
SMITHSONIAN INSTITUTION PRESS City of Washington
1983
A B S T R A C T
Menez, Ernani G., Ronald C. Phillips, and Hilconida P. Calumpong. Sea- grasses from the Philippines. Smithsonian Contributions to the Marine Sciences, number 21, 40 pages, 26 figures, 1983.—Seagrasses were collected from various islands in the Philippines during 1978-1982. A total of 12 species in seven genera are recorded. Generic and specific keys, based on vegetative characters, are provided for easier differentiation of the seagrasses. General discussions of seagrass biology, ecology, collection and preservation are presented. Local and world distribution of Philippine seagrasses are also included.
OFFICIAL PUBLICATION DATE is handstamped in a limited number of initial copies and is recorded in the Institution's annual report, Smithsonian Year. SERIES COVER DESIGN: Seascape along the Atlantic coast of eastern North America.
Library of Congress Cataloging in Publication Data Menez, Ernani G.
Seagrasses from the Philippines.
(Smithsonian contributions to the marine sciences ; no. 21) Bibliography: p.
Supt. of Docs, no.: SI 1.41:21
1. Seagrasses—Philippines. I. Phillipps, Ronald C. II. Calumpong, Hilconida P. III. Ti- tle. IV. Series.
QK495.A14M46 1983 584.73 83-600168
Contents
Page
I n t r o d u c t i o n 1 Acknowledgments 3
Materials a n d M e t h o d s 3 Collecting a n d Preserving Seagrasses 4
General Features of Seagrass Biology a n d Ecology 6
Key to t h e Philippine Seagrasses 7
Division ANTHOPHYTA 8 C l a s s MONOCOTYLEDONEAE 8
O r d e r H E L O B I A E 8 F a m i l y POTAMOGETONACEAE 8
Cymodocea rotundata E h r e n b e r g a n d H e m p r i c h , ex Ascherson 8 Cymodocea serrulata (R. Brown) Ascherson a n d M a g n u s 8
Halodule pinifolia (Miki) den H a r t o g 13 Halodule uninervis (Forskal) Ascherson 13 Syringodium isoetifolium (Ascherson) D a n d y 18 Thalassodendron ciliatum (Forskal) den H a r t o g 18
F a m i l y HYDROCHARITACEAE 23 Enhalus acoroides (L. f.) Royle 23 Halophila beccarii Ascherson 24 Halophila minor (Zollinger) d e n H a r t o g 26
Halophila ovalis (R. Brown) Hooker f. 30 Halophila spinulosa (R. Brown) Ascherson 33
Thalassia hemprichii (Ehrenberg) Ascherson 33
Literature Cited 39
i n
Seagrasses from the Philippines
Ernani G Menez, Ronald C. Phillips and Hilconida P. Calumpong
Introduction
T h e seagrass flora of t h e western tropical Pa- cific is unusually diverse (den H a r t o g , 1970).
T s u d a , Fosberg, a n d Sachet (1977) reported ten species for Micronesia; J o h n s t o n e (1979) listed 13 t a x a for P a p u a New Guinea; a n d den H a r t o g (1970) reported 11 species for the Phillipines.
Despite the interesting questions associated with this diversity a n d t h e widespread occurrence of seagrasses in the Philippines, only a few papers have been published which treat Philippine sea- grasses. Earlier records include Blanco's (1837,
1845, 1879) reports of Vallisneria sphaerocarpa ( = Enhalus acoroides) from Zambales. A n o t h e r report of Vallisneria sphaerocarpa from P a l a w a n was by Merrill (1918). Ostenfeld (1909) recorded Halo- phila ovata ( = Halophila minor) from the Philip- pines, based on Loher's specimen from Luzon a n d later, Merrill's collection from M a n i l a Bay.
A critical morphological study of Thalassia hem- prichii was published by Pascasio a n d Santos
(1930). D o m a n t a y (1962) listed eight species of seagrasses in his study of the m a r i n e vegetation of the H u n d r e d Islands in P a n g a s i n a n . Merrill (1912, 1915, 1918, 1925), M e n d o z a a n d del R o - sario (1967) included seagrasses in vascular p l a n t floras. C a l u m p o n g (1979) reported three sea- Emani G. Menez, Smithsonian Oceanographic Sorting Center, Na- tional Museum of Natural History, Smithsonian Institution, Wash- ington, D.C. 20560. Ronald C. Phillips, School of Natural and Mathematical Sciences, Seattle Pacific University, Seattle, Washing- ton, 98119. Hilconida P. Calumpong, Department of Biology, Silli- man University, Dumaguete City, Negros Oriental 6501, Philippines.
grasses in an ecological study of the sea h a r e , Dolabella auricularia (Lightfoot), in C e n t r a l V i - sayas region. Cordero (1981) illustrated a n d de- scribed the morphology a n d distribution of three species of seagrasses.
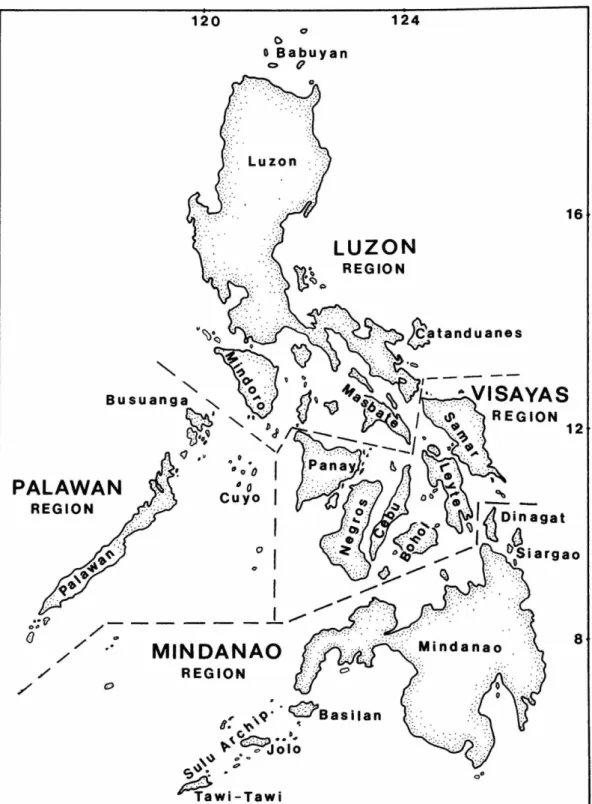
T h e Philippines is a n archipelago of a b o u t 7,100 islands. It lies between latitudes 4 ° 4 0 ' - 21°50' N a n d longitudes 1 1 6 ° 5 0 ' - 1 2 6 ° 3 5 ' E, sur- r o u n d e d by the South C h i n a Sea in t h e west, Sulu Sea a n d Celebes Sea in t h e south, t h e Phil- ippine Sea, which opens into the Pacific O c e a n , in the east, a n d t h e Luzon Strait in t h e n o r t h . T h e country is naturally divided into four geo- graphical regions (Figure 1): t h e Luzon Region, including t h e islands of Luzon, B a b u y a n , C a t a n - duanes, M i n d o r o , M a s b a t e , R o m b l o n , a n d M a r - i n d u q u e ; the M i n d a n a o region, including Min- d a n a o , Basilan, a n d the Sulu G r o u p ; t h e Visayas region, including Samar, Leyte, Bohol, C e b u , Negros, a n d P a n a y ; a n d the P a l a w a n region, including P a l a w a n , Balabac, Culion, a n d t h e Cuyo G r o u p .
Seagrasses are i m p o r t a n t , b u t their role has often been overlooked d u e largely to their sub- merged state. T h a y e r , Wolfe, a n d Williams (1975) gave an overall s u m m a r y of t h e impor- tance of seagrasses:
1. Seagrass has a high growth rate, p r o d u c i n g an average of a b o u t 300-600 g dry w e i g h t / m2/ year, not including root p r o d u c t i o n . C o m - p a r e d to world averages for cultivated corn (412 g C / m2) or rice (497 g C / m2) , seagrass beds are more productive.
1
SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCES
1 2 0 1 2 4
o B a b u y a n o o
C a t a n d u a n e s
PALAWAN
R E G I O N
16
VISAYAS
R E G I O N 12
D i n a g a t V S i a r g a o
MINDANAO
R E G I O N
„' \9' ^ • ' B a s i l a n a. <*r '•
T a w i - T a w i , * \
FIGURE 1.—General map of the Philippines.
NUMBER 21
2. The leaves support a large number of epiphy- tic organisms, with a total biomass perhaps approaching that of the seagrass itself.
3. Although a few organisms may feed directly on the seagrass and several may graze on the epiphytes, the major food chains are based on seagrass detritus and its resident microbes.
4. The organic matter in the detritus and in decaying roots initiates sulfate reduction and maintains an active sulfur cycle.
5. Seagrass roots bind the sediment together and, with the protection afforded by the leaves, surface erosion is reduced, thereby preserving the microbial flora of the sediment and the sediment-water interface.
6. Seagrass leaves retard the currents and in- crease sedimentation of organic and inorganic materials around the plants.
7. Seagrass absorbs phosphorus through the roots and the leaves; it may be that the phosphorus absorbed through the roots is released through the leaves, thereby returning phosphate from sediments to the water column. Nitrogen also is taken up by the roots and transferred to the leaves and into the medium.
It is now imperative that we properly recognize how they contribute to the oceanic realm of the Philippines. All over the world humans and sea- grasses are coming into conflict for space along the coast in areas that are in demand for human activities, from the creation of recreational places, such as marinas, to industrial ones, such as power plants, mining sites, freighter and tanker termi- nals. Unfortunately, discharges of warm water from power plants and silt and nutrients from mining, sewage treatment plants, and tanker ter- minals directly harm seagrasses. Before we de- stroy or even degrade any more seagrass habitats, we must know where the seagrasses are and the true value of this coastal resource.
The resolution of this conflict need not be an inevitable loss of seagrasses. It is our hope that this study not only will alert people to the sea- grasses by allowing them to identify the various species, but also by making them aware of where to find them and to appreciate some of the plant and animal associations that occur within the
seagrass ecosystems. Most importantly, seagrass communities in the Philippines serve as habitats and breeding grounds for various marine orga- nisms that are economically important to the local populace. Appropriate planning and reason- able environmental management can ensure the perpetuation of these ecosystems.
ACKNOWLEDGMENTS.—We wish to thank Dr.
Angel Alcala, Acting President, Silliman Univer- sity, for allowing us to use the SU Marine Labo- ratory for our work and for providing accommo- dations. We appreciate the local transportation and accommodations provided by the University of San Carlos, through Prof. Cristobal Plateros, Chairman, Department of Biology.
Hilconida Calumpong acknowledges the assist- ance of Mr. Willy Rasay and Ms. Janet Estacion in collecting and preparation of herbarium spec- imens of seagrasses. Ronald Phillips appreciates the financial aid, given by the Seattle Pacific University Institute for Research, for travel in March 1982. A portion of this research was sup- ported by the National Science Foundation, International Decade of Ocean Exploration, Liv- ing Resources Program, Seagrass Ecosystem Study (OCE77-25559). Ernani Menez appreci- ates the travel and research support in the Phil- ippines from the Smithsonian Institution Fluid Research Fund.
For their critical review of this paper, we are indebted to Dr. Raymond Fosberg, Botanist Emeritus, Smithsonian Institution, to Dr. C. den Hartog, Catholic University, Nijmegen, The Netherlands, and to Dr. Calvin McMillan, Pro- fessor of Botany, University of Texas at Austin.
The loan of Halophila beccarii and H. spinulosa specimens from Rijksherbarium, Leiden, is grate- fully acknowledged.
MATERIALS AND METHODS.—The
seagrasses col-
lected during the Smithsonian Institution Biolog-
ical Expeditions of 1978 and 1979 to Central
Visayas, Philippines, and the authors' personal
collections during 1980-1982 from the Visayas
region, Palawan, La Union, and Ilocos Norte,
Philippines, were used in this study. Replicates of
material are being deposited in the U.S. National
Herbarium (Smithsonian Institution), the Seattle
SMITHSONIAN CONTRIBUTIONS T O T H E MARINE SCIENCES
Pacific University H e r b a r i u m , the Silliman U n i - versity H e r b a r i u m , the National H e r b a r i u m of t h e Philippines, a n d the Rijksherbarium.
In the systematic section of this p a p e r , t h e a u t h o r , date, a n d page of t h e original publication of each taxon, plus basionyms a n d published records from the Philippines, are given. T h e char- acteristics, n a t u r a l history, local a n d world distri- b u t i o n of each species are also given. A dichoto- mous key a n d line drawings are included in order to facilitate easier identification of the twelve species of seagrasses from the Philippines. Most of the n a t u r a l history a n d distribution d a t a of Philippine seagrasses in this study are from den H a r t o g ' s (1964, 1970) publications. Additional information has been taken from Isaac (1968), K a y (1971), K i r k m a n (1975), M c M i l l a n (1980a, b), M c M i l l a n , Bridges, Kock, a n d F a l a n r u w (1982) a n d J o h n s t o n e (1982).
COLLECTING AND PRESERVING SEAGRASSES.—
Seagrasses m a y be collected as drift plants cast ashore or as freshly d u g specimens. T h e major problems in the use of drift plants is t h a t they m a y be broken a n d their original a t t a c h m e n t site is uncertain. It is known t h a t seagrass plants are b u o y a n t a n d can float for weeks if detached from the b o t t o m .
T h e r e c o m m e n d e d procedure for collecting a n d preserving seagrass specimens for h e r b a r i u m storage a n d study is as follows. A collector should get the entire p l a n t including the root a n d rhi- zome system. O n e should use a shovel or dig by h a n d deeply enough into the substrate to get below the rhizome mat. T h e plant mass can then be torn loose from the bottom. All sediment a n d animals should be washed from the rhizome a n d roots. Specimens covering all development phases should be collected from the same area. It is best to include plants with flowers, fruits, a n d seeds, as well as vegetative plants with intact leaf tips a n d root systems.
T h e plants should be returned to the laboratory in a plastic bag, preferably in an ice chest. T h e plants should be kept moist a n d should not be exposed to direct sunlight.
T h e plants can be preserved by d r y - m o u n t i n g on h e r b a r i u m p a p e r or by soaking first in a
solution of seawater formalin. Soaking t h e p l a n t s first in formalin keeps down t h e growth of fungi (mildew) a n d destruction by insects, b u t renders the plants useless for chemical analysis. If one wishes to kill t h e plants before m o u n t i n g on h e r b a r i u m p a p e r , the plants should b e soaked for one h o u r in a 5% solution of seawater-formalin (8 parts seawater to 1 part formaldehyde). T h e n the plants should be d r a i n e d dry a n d m o u n t e d on h e r b a r i u m sheets or other stiff, high-quality paper.
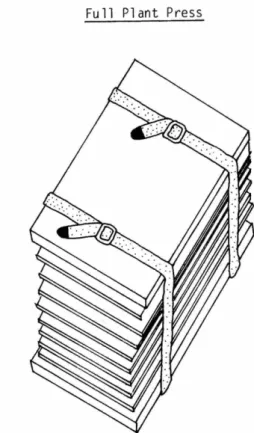
Seagrasses, such as t h e small a n d fragile Halo- phila species, are most easily m o u n t e d on herbar- ium sheets if they are floated first in a tray of seawater. A sheet of h e r b a r i u m p a p e r is placed u n d e r the p l a n t in the water, the p l a n t a r r a n g e d on the sheet, the sheet is lifted out a n d t h e excess water drained off. T h e sheet is then ready for the plant press (Figure 2).
T h e plant press can be m a d e from two pieces of 3/8-inch plywood, each 12 X 17 inches. Inside the press there should be a c o m p l e m e n t of cor- rugated c a r d b o a r d ventilators, heavy blotters, a n d waxed paper, in addition to the h e r b a r i u m p a p e r with the specimens. Each of these sheets is the same size as the p l a n t press. T w o or more straps (belts or pieces of clothesline) are needed to hold the press together.
T h e plant press is assembled by placing a h e r b a r i u m sheet with its p l a n t specimen on a blotter. A sheet of waxed p a p e r is placed on top of the specimen. A n o t h e r blotter is placed on top of the waxed paper. C a r d b o a r d ventilators are used to separate blotters. W h e n all of the plants have been p u t into the press, the straps are used to b i n d the press tightly together. T h e press m a y be placed over a source of mild heat to allow a current of w a r m dry air to rise t h r o u g h the cor- rugations of the ventilators. U n t i l t h e plants are dry, the press should be o p e n e d each day a n d dry ventilators a n d blotters exchanged for the wet ones. W h e n the sheets are dry, the dried plants should be fixed to the h e r b a r i u m sheets using clear D u c o cement.
T h e following information should b e typed on a specimen-label form: scientific n a m e (with au- thor), h a b i t a t information (depth of water, type
N U M B E R 21
Genus/Species Habitat Data:
Locati on:
Date:
Collector:
Full Plant Press
Herbarium Sheet with Data
{•>S/S//'>->f/'//Sf / / f f ^ZZ
S S S S S S S S S S S S 5 ^ ^ \ \ \ \ ^ ^ i
C
Plywood
Cardboard Ventilator Blotter
Waxed Paper
Herbarium Sheet with Specimen Blotter
Cardboard Ventilator P lywood
Plant Press Arrangement
FIGURE 2.—Illustration showing proper plant press arrangement for drying seagrasses.
SMITHSONIAN CONTRIBUTIONS TO THE MARINE SCIENCES
of substrate, etc.), location (specific but also gen- eral area), date of collection, collector's name.
The label should be pasted in the lower right corner of the sheet.
General Features of Seagrass Biology and Ecology
The narrow fringe of the edge of the sea is both the most productive and the most threatened portion of the ocean. In this area of the shallow water light can penetrate to the bottom and nutrients arrive from both land and sea. It is here that marine plant ecosystems flourish. These ma- rine systems are the foundations of inshore pro- ductivity and form the basis for animal life.
The value and significance of the shallow-water habitats have been overlooked in the past, in part because the connections from the plants to ani- mals are complex and often indirect. Paradoxi- cally, the most important contribution of all sea- grass ecosystems to the chains of life is death. The bacteria that devour the dead leaves of the plant not only nourish and enrich the area in which the plants grow, but also affect waters for miles around. Seagrass and mangrove leaves have been found in deep-sea trenches far from shore and thousands of feet deep. Even in these most remote regions of the sea, the leaves provide food and shelter for resident animals.
Seagrasses are not true grasses, but they do resemble them and have many similarities with them in the biology of their life cycle. Seagrasses usually form dense meadows that resemble more familiar prairies or grasslands. Seagrasses are vas- cular plants. The vascular system in the seagrasses consists of an internal network of tubes that is continuous from the roots to the leaf tips, trans- porting water, nutrients, and gases for tissue growth. Because of this system, seagrass growing in sand or mud can use the highly concentrated nutrients of the bottom, nutrients that may be hundreds or thousands of times more concen- trated than those in the overlying water column.
This source, the bottom sediment, is unavailable to all other marine plants; these others must depend for growth only on nutrients dissolved in the water. Seagrasses can absorb nutrients from
water through their leaves like algae, but are not limited to that source. Seagrasses, then, are a mechanism for returning nutrients to other ma- rine life that would otherwise be lost in the sedi- ments. They are the only plants in the sea that can do this. The significance of this dual nutrient source is reflected in growth rates that are high or higher than those of any other plant commu- nity in the sea. The roots and rhizomes not only absorb nutrients from the sediment, but also pre- vent the movement of bottom sediments. In dense meadows of Thalassia hemprichii (turtle grass) and
Enhalus acoroides, the roots and rhizomes form adense mat that prevents erosion of the coast that would otherwise occur from strong currents and severe typhoons. Since they occur inshore of the coral fore-reef, they act in concert with the reef in coastal protection.
Part of the importance of seagrasses is their high productivity. Seagrass beds have been shown to be among the most productive areas of the earth, including those under intensive agricul- ture. Seagrasses have a complement of attached algae, called epiphytes, which contribute to the high productivity of the seagrass ecosystem. This primary productivity is the first link in the food chains of these ecosystems, and generally supports a high productivity in the associated animal com- munity.
Seagrasses constitute a haven and refuge for animals in the sea. They are especially important as nurseries for fish, shrimps, lobsters, scallops, and other valuable food animals. The protection that these plant systems afford extends beyond their associated animal life to the coast itself.
Although many of the animal associations with seagrasses are indirect and inconspicuous, there are notable exceptions: dugongs, sea turtles, some fish, and sea urchins and other invertebrates feed directly on these plants.
A seagrass meadow or bed may consist of a few hundred to a few thousand leafy shoots per square meter and can cover bottom areas from a few acres up to many square miles. These meadows are crucial habitats for a variety of marine life.
To some animals they are a refuge; to others they
are the local swim-in restaurant where a free
lunch is always available. One of the reasons for
NUMBER 21
this concentration of marine life in seagrass meadows is the great habitat complexity resulting from the dense canopy of leaves; the leaves pro- vide a surface area that can be 20 or more times greater than that of the bottom they occupy — a veritable jungle. A myriad of small animals and plants, some epiphytes and some merely clinging, can be found on seagrass leaves. These form an essential part of the complex food webs that exist in a seagrass meadow.
An essential feature of any seagrass meadow is the fate of the leaves as they age and become detached from the leaf stalk, an event similar to the autumn drop of leaves in a forest. A large proportion of these leaves sink to the bottom where they become the basis of yet another com- plex food web. Through wave action and brows- ing activities of invertebrates, the leaves break down and decompose into particulate matter that remains suspended in the water column. In so doing, they provide inorganic nutrients that are essential to the continued growth of the living seagrasses, and nourishment to a variety of bac- teria. Some of the soluble plant chemicals leach out into the surrounding water and help to feed the same bacteria. These bacteria are the food of
diverse species of small animals that are them- selves the food for slightly larger species of ani- mals and so it goes. This decomposer food chain begins with bacteria in the upper levels of bottom sediments and around the surface of the particu- late plant matter, an environment devoid of ox- ygen, but eventually it leads to the more fa- miliar larger food animals, worms, clams, shrimps and consequently to fishes. The steps in this food chain are still more obscure than the one based on direct grazers and there is still doubt about its significance in the economy of the sea. The pro- duction of detritus from the constant sloughing of leaves in the seagrass meadow results in a relatively constant source of food for the bacteria and animals of the decomposer food chain throughout the year.
Many studies in the United States have proven that seagrass presence and growth is absolutely dependent on relatively clear water. Seagrasses stabilize bottom sediments and baffle water mo- tion inside the meadow, but additions of dirty water from dredging, mining, sewage, and boat- ing activities can sharply curtail or eliminate the productivity or even presence of seagrass in an
Key to the Philippine Seagrasses
Leaves terete Syringodium isoetifolium
Leaves flat 2 Leaves elliptic, ovate, lanceolate or linear-oblong; 4—30 mm long
Halophila
A. Leaves lanceolate, without cross-veins H. beccarii
Leaves otherwise, with cross-veins B B. Leaves with 12-22 pairs of cross-veins H. ovalis
Leaves with 4-7 pairs of cross-veins C C. Leaves up to 22 pairs, distichous on long shoot H. spinulosa
Leaves in pairs on every short shoot H. minor
Leaves linear, usually 5-100 cm long 3
Leaves ligulate 4 Leaves not ligulate 6 Nerves no more than 3 Halodule
A. Leaf tip with irregular serrations; tip of midrib often furcate
H. pinifolia
Leaf tip tridentate; tip of midrib not furcate H. uninervis
Nerves more than 3 5
SMITHSONIAN CONTRIBUTIONS TO THE MARINE SCIENCES
5. U n b r a n c h e d or once-branched shoots p r o d u c e d at every fourth i n t e r n o d e on rhizome; leaf tips with bi- trifurcate teeth; roots b o r n e o n i n t e r n o d e with n u m e r o u s t o u g h , wiry laterals; internodes 3 - 1 0 m m long
Thalassodendron ciliatum
Not as above Cymodocea A. Leaf t i p smooth or slightly serrulate; leaf blades linear C. rotundata
Leaf tip serrate-dentate; leaf blades linear-falcate C. serrulata 6. R h i z o m e 2 - 4 m m in diameter; roots densely beset with filiform laterals;
leaves linear-falcate; w i t h o u t basal black fibers .. Thalassia hemprichii R h i z o m e m o r e t h a n 1 c m in diameter; roots naked; leaves linear; with
persistent basal black fibers Enhalus acoroides Division A N T H O P H Y T A
C l a s s MONOCOTYLEDONEAE
Order H E L O B I A E
Family P O T A M O G E T O N A C E A E
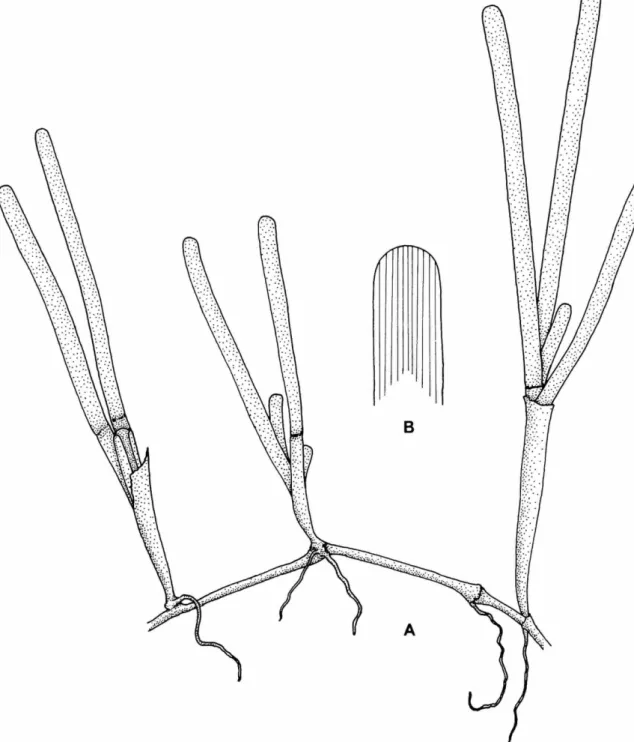
Cymodocea rotundata Ehrenberg and Hemprich, ex Ascherson
FIGURES 3A,B, 4
Cymodocea rotundata Ehrenberg and Hemprich, ex Ascherson, 187la:83.—Merrill, 1925:24.—Mendoza and del Rosario, 1967:12.—den Hartog, 1970:166, fig. 47.
CHARACTERISTICS.—Plants of m o d e r a t e size, with moderately robust rhizomes, reaching 2 m m in diameter; internodes 0 . 5 - 3 c m long; laxly b r a n c h e d roots usually 1-3 p e r node. Erect shoots short, generally with 2 - 4 leaves. Persistent leaf sheaths u p to 4 cm long, usually forming scarious mass at m a t u r i t y ; w h e n shed, conspicuous scars are left o n stem. Leaf blades linear, 2 - 4 m m wide a n d u p to 10 c m long, with r o u n d apices or slightly serrulate, nerves 8-12.
NATURAL HISTORY.—Cymodocea rotundata occurs in shallow water, on s a n d - m u d or sand substrates in sheltered coves or bays, lagoons, m o u t h of rivers a n d coral reefs. T h e p l a n t m a y b e found growing with Halophila ovalis, Halodule uninervis, Enhalus acoroides, Thalassia hemprichii, Syringodium isoetifolium, or Cymodocea serrulata. E p i p h y t i c algae found on leaves of t h e p l a n t include melobesioids a n d Hypnea. In N e w C a l e d o n i a a n d T h u r s d a y
Island, s t a m i n a t e flowers were observed d u r i n g April a n d November, respectively. Pistillate flow- ers h a v e been reported d u r i n g N o v e m b e r a t T h u r s d a y Island. At Bristow Island, b o t h stami- n a t e a n d pistillate flowers were found in M a r c h . Plants with fruits were recorded from Y a p , Tomil-Gagil, a n d M a p islands in Micronesia dur- ing M a r c h a n d J u n e - J u l y . I n N e w G u i n e a a n d t h e island of Bali, fruits were observed in J u n e .
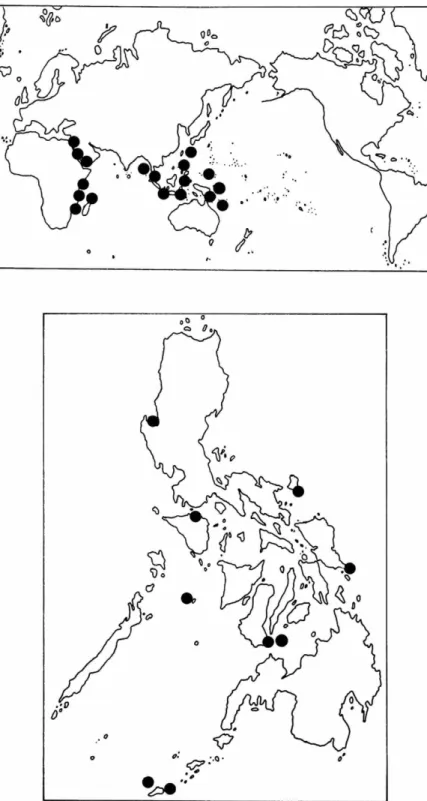
PHILIPPINE D I S T R I B U T I O N . — L u z o n (Lingayen Gulf, C a t a n d u a n e s , M i n d o r o ) ; P a l a w a n (Cuyo Islands); Visayas (Samar, Negros, Siquijor); M i n - d a n a o (Pearl Bank, T a w i - T a w i G r o u p ) .
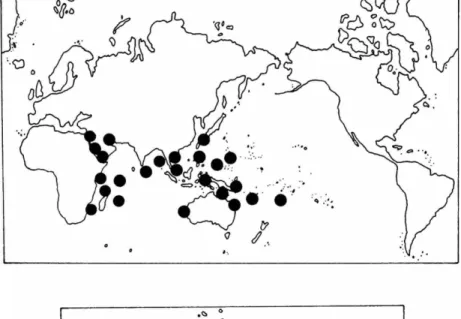
R A N G E . — C . rotundata is widely distributed in t h e I n d i a n a n d western Pacific oceans. It occurs in Egypt, S u d a n , K e n y a , T a n z a n i a , M o z a m - b i q u e , M a d a g a s c a r , Y e m e n , T h a i l a n d , A n d a m a n Islands, R y u k y u Islands, Malaysia, Indonesia, P a p u a N e w Guinea, Caroline Islands, Queens- land, a n d New Caledonia.
Cymodocea serrulata (R. Brown) Ascherson and Magnus
FIGURES 5 A - C , 6
Caulinia serrulata R. Brown, 1810:339.
Cymodocea serrulata (R. Brown) Ascherson and Magnus, in Ascherson, 187la:84.—Merrill, 1925:24.—Mendoza and del Rosario, 1967:13.—den Hartog, 1970:171, fig. 48.
CHARACTERISTICS.—Plants of m o d e r a t e size, rhizomes 2 - 3 m m in d i a m e t e r ; internodes 1-4 c m long; usually 2 to several sparsely b r a n c h e d roots per node. Erect shoots short, b e a r i n g u p to 4
NUMBER 21
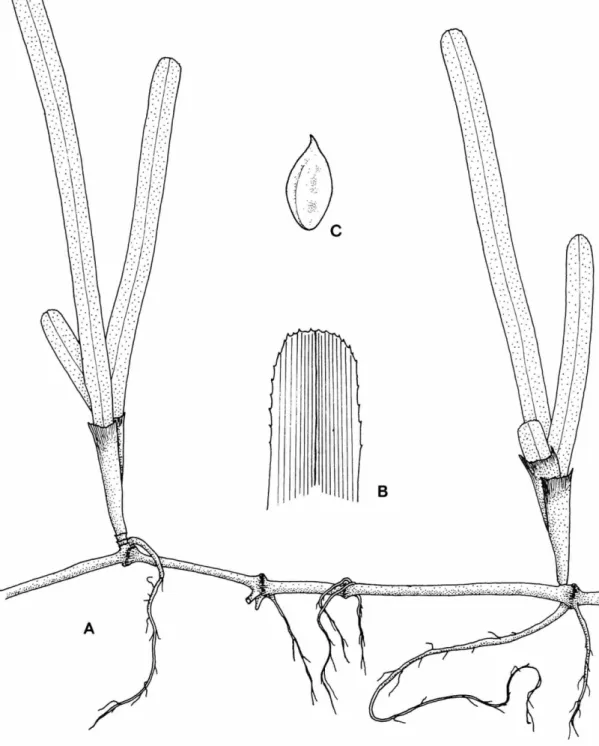
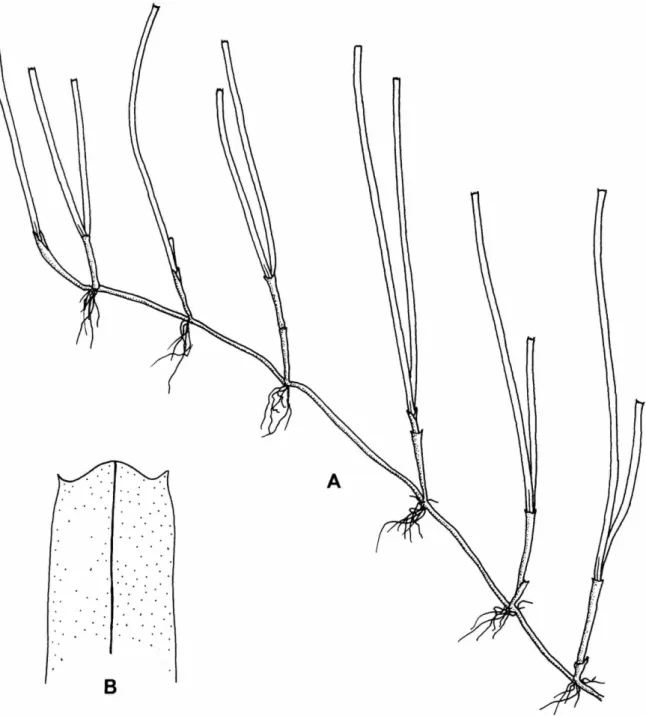
B
FIGURE 3.—Cymodocea rotundata: A , habit of plant, X 1.4; B, upper portion of a leaf showing perpendicular veins, X 4.4.
10 SMITHSONIAN CONTFIBUTIONS TO THE MARINE SCIENCES
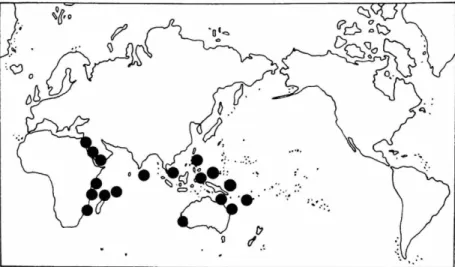
FIGURE 4.—Cymodocea rotundata: top, world distribution; bottom, Philippine distribu- tion.
NUMBER 21
11
FIGURE 5.—Cymodocea serrulata: A, habit of plant, X 1.2; B, upper portion of a leaf showing serrate tip and perpendicular veins, X 2.2; c, fruit, X 1.8
12
SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCESFIGURE 6.—Cymodocea serrulata: top, world distribution; bottom, Philippine distribu- tion.
NUMBER 21 13 leaves. Leaf sheaths 1-3 c m long, t a p e r e d towards
t h e base; w h e n shed, leaving conspicuous scars a r o u n d t h e stem. Leaf blades linear-falcate, 3-7 m m wide, u p to 9 c m long, n a r r o w e d at t h e base;
with r o u n d to obtuse, serrate leaf tips; margins mostly entire, spinulose only n e a r t h e t i p ; h a v i n g 8-16 nerves connected by cross-veins. Fruits nearly elliptic, compressed, sessile a n d with dorsal ridges.
NATURAL HISTORY.—Cymodocea serrulata occurs in t h e lower intertidal zone, in sheltered localities, on coarse coral-sand, s a n d - m u d , or m u d with coral r u b b l e substrates. T h e plant is reported growing together with Thalassia hemprichii, Enhalus acoroides, Halophila ovalis, Halodule uninervis, a n d Syringodium isoetifolium. Pistillate flowers have been reported d u r i n g April in New Caledonia; in K e n y a , they have been observed in J a n u a r y a n d August. Plants with s t a m i n a t e flowers have been recorded d u r i n g F e b r u a r y a n d M a r c h from M o r - eton Bay, Q u e e n s l a n d . In K e n y a , they occurred in September. C o n t r a r y to d e n H a r t o g ' s (1970) c o m m e n t t h a t Cymodocea serrulata is absent in places where there is freshwater influence, in Albay Gulf, southeastern Luzon, Philippines, t h e p l a n t was collected from boulders n e a r t h e m o u t h of a stream, growing together with Halodule uni- nervis. E p i p h y t i c algae found on t h e leaves of Cymodocea serrulata were melobesioids, Ceramium a n d Acrochaetium. I n Negros, Philippines, fruits were found on Cymodocea serrulata in August.
PHILIPPINE D I S T R I B U T I O N . — L u z o n (Pangasi- n a n , Albay, C a t a n d u a n e s , M i n d o r o ) ; P a l a w a n (Bajallanura Island, D o u b l e Island); Visayas (Ne- gros, Leyte); M i n d a n a o ( Z a m b o a n g a ) .
R A N G E . — C . serrulata is c o m m o n in tropical a n d subtropical regions. It occurs in Egypt, S u d a n , K e n y a , T a n z a n i a , M o z a m b i q u e , M a d a g a s c a r , C o m o r o Islands, Seychelles, Yemen, Sri L a n k a , Malaysia, Indonesia, Western Australia, Queens- land, P a p u a N e w Guinea, Caroline Islands, a n d New Caledonia.
Halodule pinifolia (Miki) den Hartog
FIGURES 7A,B, 8
Diplanthera pinifolia Miki, 1932:787.
Halodule pinifolia (Miki) den Hartog, 1964:309; 1970:158.
CHARACTERISTICS.—Plants small, with slender rhizomes, u p to 1.5 m m in diameter; internodes usually 0.5-2.0 c m long; simple or laxly b r a n c h e d roots, 2 to several per node. Short, erect shoots consisting of 2 - 4 leaves b o r n e at each node. Leaf blades linear, 4 - 1 5 c m long, not m o r e t h a n 1.5 m m wide. Leaf tips obtuse with few irregular serrations. Leaf margins entire; conspicuous mid- rib furcate at t h e tip. Basal portion of leaves enclosed by sheaths, 3 c m long. O v a t e scales on each node not persistent at m a t u r i t y .
NATURAL HISTORY.—Halodule pinifolia occurs in sheltered a n d semi-exposed bays, coral reef plat- forms, in pools, also in wave-beaten sites. T h e plant has only been collected from two sites along t h e coast of Negros a n d S a m a r islands of t h e Visayas region of the Philippines. T h o s e collected from Negros were from t h e u p p e r sublittoral zone a n d were sterile. T h e rarity of this species in o u r collections suggests t h a t it is less tolerant of pre- vailing h y d r o g r a p h i c p a r a m e t e r s c o m p a r e d to other seagrasses. In t h e literature (den H a r t o g , 1970), its c o m p a n i o n species on c o m p a c t m u d is Cymodocea rotundata a n d on soft m u d , Halophila ovalis. At Cairns, Q u e e n s l a n d , flowering a n d fruit- ing plants of Halodule pinifolia were found d u r i n g November. Species of crustose melobesioids Acro- chaetium, Ceramium, Hypnea, a n d Polysiphonia, were found growing on leaves of Halodule pinifolia.
PHILIPPINE D I S T R I B U T I O N . — L u z o n ( M i n d o r o ) ; Visayas (Negros, S a m a r , Bohol); M i n d a n o (Cav- illi Island, Pearl Bank).
R A N G E . — H . pinifolia is most c o m m o n l y distrib- u t e d in tropical a n d subtropical localities. It oc- curs in R y u k y u Islands, V i e t n a m , Indonesia, T o n g a Islands, P a p u a N e w Guinea, Fiji Islands, Q u e e n s l a n d a n d N e w Caledonia.
Halodule uninervis (Forskal) Ascherson
FIGURES 9A,B, 10
Zostera uninervis Forskal, 1775:157.
Halodule uninervis (Forskal) Ascherson, in Boissier, 1882:24.—
den Hartog, 1970:147.
Diplanthera uninervis Ascherson, in Engler and Prantl, 1897:37.—Domantay, 1962:275.—Mendoza and del Ro- sario, 1967:13.
CHARACTERISTICS.—Plants of m o d e r a t e size,
14 SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCES
FIGURE 7.—Halodule pinifolia: A, habit of plant, X 2.1; B, upper portion of leaf showing furcate midrib and irregular teeth at tip, X 28.
NUMBER 21 15
FIGURE 8.—Halodule pinifolia: top, world distribution; bottom, Philippine distribution.
16
SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCESFIGURE 9.—Halodule uninervis: A, habit of plant, X 0.95; B, leaf tip showing two acuminate teeth and median nerve, X 10.4.
with slender rhizomes barely reaching 2 mm in diameter; internodes 2-3 cm long; unbranched to sparsely branched roots not more than 5 per node.
Erect shoots short, with 2-4 leaves enveloped
below by a 1-2 cm long leaf sheath; when shed,
leaf sheaths leave conspicuous annular scars on
stem. Leaf blades linear and narrow at the base,
2-5 mm wide, reaching 15 cm in length. Blades
NUMBER 21
17
FIGURE 10.—Halodule uninervis: top, world distribution; bottom, Philippine distribu- tion.
1 SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCES with 3 nerves; t h e m i d d l e o n e very distinct a n d
e n d i n g in t h e m e d i a n apical obtuse tooth, while t h e lateral nerves e n d i n g in 2 a b r u p t l y a c u m i n a t e teeth. Leaf tip t r i d e n t a t e ; leaf m a r g i n entire.
NATURAL HISTORY.—Halodule uninervis occurs in sheltered or exposed localities in t h e shallow in- tertidal to t h e u p p e r subtidal zone, on s a n d - m u d , coarse coral-sand, or sand substrates. In t h e Phil- ippines, occasional patches of t h e p l a n t m a y b e mixed with Thalassia hemprichii, Halophila ovalis, Syringodium isoetifolium, Enhalus acoroides, a n d Cy- modocea serrulata. O n t h e leaves a n d particularly t h e basal parts of Halodule univervis, epiphytic algae, such as Colpomenia sinuosa, Dictyopteris deli- catula, Jania adherens, Mastophora rosea, a n d species of Ceramium, were found. Flowering a n d fruiting plants of Halodule uninervis h a v e not been observed to d a t e in t h e Philippines. At Q u e e n s l a n d , flow- ering plants were found d u r i n g O c t o b e r a n d N o - vember.
P H I L I P P I N E D I S T R I B U T I O N . — L u z o n ( M a n i l a Bay, P a n g a s i n a n , Albay, C a t a n d u a n e s , M i n d o r o , L a U n i o n , Ilocos Norte); P a l a w a n (Cuyo Islands);
Visayas (Negros, Bohol, Leyte, C e b u ) ; M i n d a n o ( Z a m b o a n g a ) .
RANGE.—Halodule uninervis is widely distributed along t h e tropical coast of t h e I n d i a n a n d western Pacific oceans. It occurs in Egypt, Eritrea, So- malia, T a n z a n i a , M a d a g a s c a r , M o z a m b i q u e , M a u r i t i u s , Seychelles, Saudi Arabia, K u w a i t , B a h r a i n Islands, Yemen, Sri L a n k a , A n d a m a n Islands, T h a i l a n d , V i e t n a m , Malaysia, Indonesia, P a p u a N e w Guinea, Western Australia, Queens- land, N e w Caledonia, T o n g a Islands, Caroline Islands, M a r i a n a Islands, a n d J a p a n .
Syringodium isoetifolium (Ascherson) Dandy
FIGURES 11A-D, 12 Cymodocea isoetifolia Ascherson, 1868:163.
Syringodium isoetifolium (Ascherson) Dandy, in Dandy and Tandy, 1939:116.—den Hartog, 1970:177, figs. 50, 51a-d.
CHARACTERISTICS.—Plants of m o d e r a t e size, with slender rhizomes not m o r e t h a n 1.5 m m in diameter; internodes 3-15 m m long; one to sev- eral laxly b r a n c h e d roots below t h e erect shoots.
Erect shoot consisting of 2 - 3 terete leaf blades p e r node. Leaf blades reaching 25 c m long, 1.0-2.5 m m in diameter, n a r r o w towards t h e base; central vascular b u n d l e encircled b y 6 - 1 1 air channels;
pericentral vascular b u n d l e s 7-9. Leaf sheaths u p to 5 c m long. Inflorescence cymose; sheaths of reduced leaves in t h e inflorescence mostly 4 - 7 m m long. S t a m i n a t e flowers with 2 ovate anthers, 4 m m long a n d connected b y t h e m i d d l e to t h e apex of a 2 m m , terete stalk.
NATURAL HISTORY.—Syringodium isoetifolium oc- curs in sheltered a n d semi-exposed h a b i t a t s , on coarse coral-sand substrate. T h e plants are found in t h e u p p e r subtidal zone, forming thick carpets with Thalassia hemprichii, Cymodocea rotundata, C.
serrulata, a n d Halodule uninervis. In t h e Philippines, pistillate a n d s t a m i n a t e flowers were found dur- ing M a y . Crustose melobesioids sometimes heavy on leaf blades; Chaetomorpha crassa, Tolypiocladia glomerulata, Hypnea valentiae, Dictyota species, a n d
Gracilaria blodgettii a t t a c h e d at t h e base of leaves a n d on leaf sheaths of S. isoetifolium.
PHILIPPINE D I S T R I B U T I O N . — L u z o n (Pangasi- n a n , Albay, C a t a n d u a n e s , M i n d o r o , L a U n i o n ) ; Visayas (Negros, Siquijor, Bohol); M i n d a n a o
( Z a m b o a n g a ) .
RANGE.—Syringodium isoetifolium is widely dis- t r i b u t e d in t h e tropics. It occurs in Egypt, K e n y a , T a n z a n i a , M o z a m b i q u e , M a d a g a s c a r , Sey- chelles, M a u r i t i u s , Yemen, B a h r a i n Islands, Sri L a n k a , Nicobar Islands, V i e t n a m , Malaysia, P a p u a N e w G u i n e a , T o n g a Islands, Caroline Is- lands, R y u k y u Islands, Western Australia, Q u e e n s l a n d , a n d N e w Caledonia.
Thalassodendron ciliatum (Forskal) den H a r t o g
FIGURES 1 3 A - C , 1 4 Zostera ciliata Forskal, 1775:157.
Thalassodendron ciliatum (Forskal) den Hartog, 1970:186, figs.
52a-j.—Menez and Calumpong, 1983:103-111.
CHARACTERISTICS.—Plants m o d e r a t e l y large, occasionally b r a n c h e d once, r e a c h i n g a height of 45 cm. R h i z o m e thick, u p to 5 m m in diameter;
internodes 3 - 1 0 m m long; roots 1-6 b o r n e on
NUMBER 21
19
FIGURE 11.—Syringodium isoetifolium: A, habit of plant, X 0.3; B, staminate flower with 2 anthers, X 4.1; c, portion of male plant, X 3.1; D, section of leaf-blade showing central vascular bundle and air channels, X 21.8.
20 SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCES
FIGURE 12.—Syringodium isoetifolium: top, world distribution; bottom, Philippine distri- bution.
NUMBER 21
21
FIGURE 13.— Thalassodendron ciliatum: A, habit of plant, X 0.8; B, leaf with ligules and sheath, X 1.9; c, details of apical portion of leaf showing denticulate tip, irregularly serrate margin and nerves connected by cross veins, X 6.3.
22
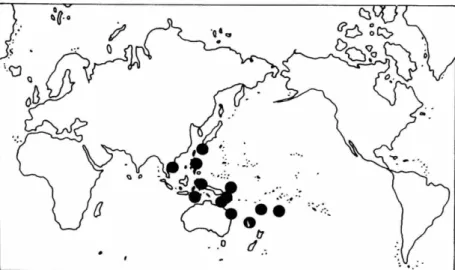
SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCESFIGURE 14.— Thalassodendron ciliatum: top, world distribution; bottom, Philippine dis- tribution.
NUMBER 21 23 internode, with t o u g h , wiry laterals, n o m o r e t h a n
1.5 m m in diameter. T w o stems p r o d u c e d a t every fourth internode o n rhizome; o n e fully de- veloped, long a n d erect with n u m e r o u s leaf scars a n d a n o t h e r , usually a n undeveloped stem-bud.
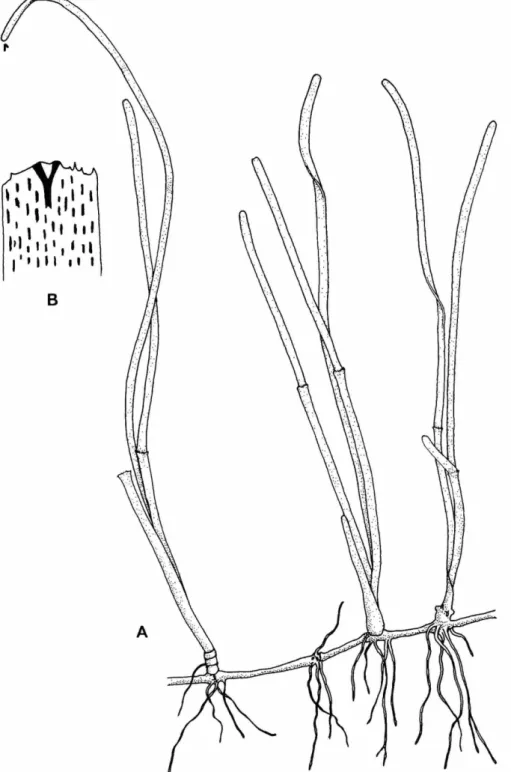
U p p e r p a r t of erect stem compressed, b u t t h e basal portion terete. Leaf blades usually 6, linear- falcate, n a r r o w e d towards t h e base, u p to 10 m m wide a n d 13 c m long; leaf tips r o u n d e d , dentic- ulate; apical teeth bi- trifurcate; u p p e r half of leaf m a r g i n with irregular serrations, becoming distant a n d few towards t h e base; lower half of leaf m a r g i n almost entire. Leaf nerves 12-18, connected by a few oblique cross-veins. Leaf sheaths obtuse, u p to 12 m m wide a n d 20 m m long, with obtuse auriculae a n d ligules.
NATURAL H I S T O R Y . — Thalassodendron ciliatum oc- curs in sheltered a n d semi-exposed localities, in the u p p e r sublittoral zone. T h e plants from t h e Philippines were sterile a n d found in small patches a m o n g dead a n d living corals in clear water. I n K e n y a a n d M o z a m b i q u e , staminate a n d pistillate flowers were observed d u r i n g Au- gust a n d J a n u a r y , respectively. In T a n z a n i a , s t a m i n a t e flowers have been recorded d u r i n g Au- gust. Algal epiphytes found growing on Philip- pine specimens of T. ciliatum include Hypnea val- entiae, Jania adherens, Amphiroa rigida, Codium arabi- cum, Giffordia rallsiae, Sphacelaria furcigera, Polysi- phonia mollis, species of Padina a n d Dictyota, Cer- amium mazatlanense, a n d Champia parvula. T h e latter two algal t a x a were present on b o t h leaves a n d stems, while t h e rest were only on t h e stem.
T h e a u t h o r s wish to point o u t t h a t Thalassoden- dron ciliatum m a y often b e confused with the "long- s t e m m e d " Cymodocea serrulata. J o h n s t o n e (1982) has found t w o growth forms of Cymodocea serrulata in N e w G u i n e a a n d reported t h a t t h e " n o r m a l "
form to b e t h e most c o m m o n . M c M i l l a n (1983) has found evidence t h a t t h e " l o n g - s t e m m e d "
plants are controlled by light intensity. T ciliatum a n d C. serrulata are easily distinguished b y a care- ful morphological examination.
P H I L I P P I N E D I S T R I B U T I O N . — P a l a w a n (Bajallan- u r a Island, T i d e p o l e Island, Double Island, west- ern side of T a b o n C a v e at Q u e z o n ) .
R A N G E . — T ciliatum occurs in Egypt, S u d a n ,
Somalia, K e n y a , T a n z a n i a , M o z a m b i q u e , S o u t h Africa, M a d a g a s c a r , Chagos Archipelago, Sey- chelles, A m i r a n t e Islands, A l d a b r a Islands, C o m - oro Island, M a u r i t i u s , Saudi A r a b i a , I r a n , I n d o - nesia, P a p u a New Guinea, Solomon Islands, C a r - oline Islands, a n d Queensland.
Family H Y D R O C H A R I T A C E A E
Enhalus acoroides (L. f.) Royle
FIGURE 15, 16
Stratiotes acoroides L. f., 1781:268.
Vallisneria sphaerocarpa Blanco, 1837:780; 1845:538; 1879:
186.—Merrill, 1918:59.
Enhalus acoroides (L. f.) Royle, 1840:453.—Merrill, 1925:27.—
Domantay, 1962:275.—den Hartog, 1970:215, fig. 60.—
Cordero, 1981:321, fig. 1.
CHARACTERISTICS.—This is t h e largest of t h e seagrass species from t h e Philippines, reaching a height of more t h a n a meter. R h i z o m e thick, u p to 1.5 c m in diameter, deeply e m b e d d e d in sub- strate, bearing m a n y roots, 2 - 5 m m in d i a m e t e r a n d u p to 15 cm long. Persistent leaf fibers very dense a n d masking rhizome. Leaves 3 or 4, pro- duced directly from rhizome; leaf blades linear, to 1.5 cm wide a n d reaching 1 m in length; leaf tips rounded, beset occasionally with m i n u t e ser- rate projections when young; leaf margins entire, slightly rolled. Characteristic tough, black vas- cular bundles persistent after d e a t h a n d decay of other leaf tissues. Nerves 10-40, parallel to a b o u t as m a n y air-channels. Fruits ovoid, 4 - 7 c m long, with a c u m i n a t e tips a n d densely covered w i t h bifid projections. Fruit stalk long a n d coiled.
NATURAL HISTORY.—Enhalus acoroides occurs in sheltered localities in t h e lower intertidal a n d u p p e r subtidal zones, on s a n d - m u d , coarse coral- sand or m u d substrates. In t h e Philippines, t h e plant was observed in p u r e stands next to a mangrove where silt was thick a n d w a t e r w a s murky, or in occasional patches in coarse coral- sand growing together with Thalassia hemprichii, or mixed with Halodule uninervis, Cymodocea serru- lata, Syringodium isoetifolium, a n d Halophila ovalis.
Fruiting plants of Enhalus acoroides were found in M a y - O c t o b e r in Central Visayas a n d d u r i n g
24 SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCES
FIGURE 15.—Enhalus acoroides: habit of plant, X 0.4.
April s t a m i n a t e flowers were collected from southern Luzon in t h e Philippines. According to den H a r t o g (1970), this p l a n t p r o b a b l y flowers and fruits t h e whole year r o u n d . Crustose melo- besioids are sometimes very thick on leaves a n d on black " h a i r s . " Hypnea a n d Cladophora were found attached to t h e melobesioids. Few thalli of Ceramium were epiphytic on Enhalus acoroides leaves.
PHILIPPINE D I S T R I B U T I O N . — L u z o n ( Z a m b a l e s , C a t a n d u a n e s , M i n d o r o ) ; P a l a w a n (Tay-tay Bay, Quezon, C u y o Islands); Visayas (Cebu, S a m a r , Leyte, Negros, Bohol); M i n d a n a o (Surigao, Z a m - boanga, Basilan).
R A N G E . — E . acoroides is widely distributed in t h e tropics. It occurs in K e n y a , .Seychelles, M a d - agascar, Saudi A r a b i a , Y e m e n , India, Sri L a n k a , A n d a m a n Islands, Malaysia, K a m p u c h e a , Viet- n a m , R y u k y u Islands, Indonesia, P a p u a N e w Guinea, M a r i a n a Islands, Caroline Islands, Queensland, a n d N e w Caledonia.
Halophila beccarii Ascherson FIGURES 17A,B, 18
Halophila beccarii Ascherson, 1871b:302.—Merrill, 1912:70;
1925:25.—Mendoza and del Rosario, 1967:21.—den Har- tog, 1970:261.
CHARACTERISTICS.—Plants small, with slender rhizomes not more t h a n 1 m m in d i a m e t e r ; inter- nodes 1-3 cm long; with one root at each node.
U n e v e n pair of scales enveloping t h e erect shoot t h a t consists of u p to 10 closely set, petiolated leaves. Leaf blades lanceolate, with acute apices a n d c u n e a t e or a t t e n u a t e bases, 8 - 1 5 m m long a n d 1-2 m m wide; margins entire, rarely mi- nutely spinulose. Conspicuous m i d r i b extending to leaf apex, with o n e i n t r a m a r g i n a l vein on each side t h a t is connected only at t h e base of t h e midrib a n d j o i n i n g it again j u s t below t h e tip.
Cross-veins absent.
NATURAL HISTORY.—Halophila beccarii occurs in the shallow, intertidal zone in sheltered localities, such as mangroves, m o u t h s of rivers, a n d brackish ponds. T h e plant seems to favor s a n d - m u d , par- ticularly m u d d y substrates. In Singapore, samples of t h e species were found a b u n d a n t on m u d a n d
NUMBER 21 25
FIGURE 16.—Enhalus acoroides: top, world distribution; bottom, Philippine distribution.
26 SMITHSONIAN CONTRIBUTIONS TO THE MARINE SCIENCES
FIGURE 17.—Halophila beccarii: A, habit of plant, X 2.9; B, leaves showing entire and spinulose margins, including conspicuous midrib and intramarginal veins, X 7.3.
on sand in shaded stream beds, a n d in brackish water in mangroves. Flowering of Halophila bec- carii have been observed d u r i n g February, J u n e , a n d August. T h e p l a n t m a y be found growing in p u r e stand or together with Halophila ovalis, H.
minor, or Halodule uninervis. A few patches of crus- tose melobesioids were observed growing on leaves of Halophila beccarii collected from the Phil- ippines.
P H I L I P P I N E D I S T R I B U T I O N . — L u z o n ( M a n i l a Bay, P a r a n a q u e ) .
R A N G E . — H . beccarii occurs in Chilka Lake, a salt-water lake along the eastern coast of India. It has also been recorded in Sri L a n k a , B u r m a , V i e t n a m , a n d Malaysia.
Halophila minor (Zollinger) den Hartog
FIGURES 19A-C, 20 Lemnopsis minor Zollinger, 1854:75.
Halophila minor (Zollinger) den Hartog, 1957:410.- and del Rosario, 1967:21.
-Mendoza
NUMBER 21
27
FIGURE 18.—Halophila beccarii: top, world distribution; bottom, Philippine distribution.
28 SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCES
FIGURE 19.—Halophila minor:A, habit of plant, X 4.9; B, leaf showing cross veins, X 9.7; c, scale, X 29.1.
NUMBER 21
29
FIGURE 20.—Halophila minor: top, world distribution; bottom, Philippine distribution.
30 SMITHSONIAN CONTRIBUTIONS T O T H E MARINE SCIENCES
Halophila ovata sensu Ostenfeld, 1909:68.—sensu Merrill, 1912:70; 1925:26.—sensu den Hartog, 1970:251. [Non Gaudichaud, 1826 [1829]: 430 (= H. ovalis (R. Brown) Hooker f.).]
CHARACTERISTICS.—This is p r o b a b l y t h e small- est of t h e seagrasses found in t h e Philippines, with rhizomes barely reaching 1 m m in diameter;
internodes 7-20 m m long; usually o n e root below each erect shoot. Erect shoot short, consisting of a pair of leaves on each node. Leaves with pe- tioles, 2 - 8 m m long, each enveloped b y a p a i r of t r a n s p a r e n t , orbicular scales with r o u n d e d apices.
Leaf blades elliptic or obovate, w i d t h 2 - 3 m m , length 4 - 7 m m , apices r o u n d e d , bases a t t e n u a t e ; margins entire; with 4 - 7 pairs of cross-veins.
S t a m i n a t e flowers pedicellate, with obtuse, con- vex tepals; anthers a p p r o x i m a t e l y 2 m m long.
Pistillate flowers with 3 styles, each u p to 15 m m long; ovary ovoid, reaching a length of 3 m m . Ellipsoid, ovoid or globose fruits u p to 4 m m long, b e a r i n g 2 - 6 m m long beaks; seeds subglobose, a b o u t 0.5 m m long.
T h e authors follow t h e remarks of Sachet a n d Fosberg (1973:441) t h a t t h e correct n a m e for this species is Halophila minor, since Halophila ovata is a superfluous n a m e , hence illegitimate.
NATURAL HISTORY.—Halophila minor occurs in sheltered coasts on m u d , sand, or coral r u b b l e substrates. T h e p l a n t seems to prefer m u d d y bot- toms a n d tolerates heavy sedimentation, as gen- erally found in deep b a y localities t h a t are well protected from wave action. In t h e Philippines, H. minor plants were collected from s a n d - m u d or coral r u b b l e substrates d u r i n g J a n u a r y - M a y . T h e y were a b u n d a n t in M a r c h - M a y a n d b e a r i n g flowers a n d fruits. Flowering plants h a v e been observed in J a n u a r y a n d in April, including fruits, a t N e w Caledonia. Plants with fruits a n d flowers were collected from K e n y a in J u l y . D e n H a r t o g (1970) suggested t h a t in areas where t h e plants are reproductive, they are a n n u a l s , a n d in places where they d o not flower, they m a y b e perennials. Halophila minor have been found grow- ing together with Halophila ovalis, Enhalus acoroides, Thalassia hemprichii, a n d Halodule uninervis. E x a m - ination of epiphytes revealed few patches of crus-
tose red algae on leaves of H. minor from t h e Philippines.
P H I L I P P I N E D I S T R I B U T I O N . — L u z o n ( M a n i l a Bay, Albay, M i n d o r o ) ; Visayas (Cebu, Negros).
RANGES.—Halophila minor occurs in K e n y a , In- dia, Singapore, Indonesia, P a p u a . N e w Guinea, Western Australia, Q u e e n s l a n d , N e w Caledonia, S a m o a , Fiji, Wallis Islands, Caroline Islands, M a r i a n a Islands, H a w a i i .
Halophila ovalis (R. Brown) Hooker f.
FIGURES 2 1 A - C , 22
Caulinia ovalis R. Brown, 1810:339.
Halophila ovalis (R. Brown) Hooker, 1858:45.—Merrill, 1925:26.—Domantay, 1962:275.—Mendoza and del Ro- sario, 1967:22.—den Hartog, 1970:240.—Cordero, 1981:
322.
Halophila ovata Gaudichaud, 1826 [1829]:430.
CHARACTERISTICS.—Plants small, with long a n d n a r r o w rhizomes, n o t m o r e t h a n 2 m m in diam- eter; internodes 5 - 4 0 m m long; usually o n e root present below each erect shoot. Erect shoot short, consisting of a p a i r of leaves b o r n e on each node.
Leaves petiolated; petioles 5 - 5 0 m m long, each enveloped by a p a i r of t r a n s p a r e n t scales; apex of scale e m a r g i n a t e , t h e base auriculate. Leaf blades oblong, oval, elliptic or s p a t h u l a t e , occasionally lanceolate or linear, w i d t h 4 - 1 2 m m , length 8 - 25 m m , apices r o u n d e d , bases c u n e a t e , a t t e n u a t e or t r u n c a t e ; margins entire; with 12-22 pairs of cross-veins which a r e often forked. M i d r i b con- nected to t h e i n t r a m a r g i n a l nerve a t t h e top.
S t a m i n a t e flowers consist of small, elliptic, convex tepals generally obtuse or slightly apiculate; an- thers oblong. Pistillate flowers with small ovoid ovaries; 3 long styles. Fruits globose with beaks u p to 6 m m long.
NATURAL HISTORY.—Halophila ovalis occurs in open or sheltered coasts, from m i d t i d e to 10 m deep in Central Philippines. T h e p l a n t grows on a wide variety of substrate, such as m u d , living corals, or coral r u b b l e , a n d in sand where t h e plant is occasionally almost completely buried.
According to t h e literature, H. ovalis is a euryha- line species t h a t c a n grow in waters with de-
NUMBER 21 31
FIGURE 21.—Halophila ovalis: A, habit of plant, X 2.2; B, leaf showing cross veins, X 5.6; c, scale, X 10.4.
32 SMITHSONIAN CONTRIBUTIONS TO T H E MARINE SCIENCES
FIGURE 22.—Halophila ovalis: top, world distribution; bottom, Philippine distribution.
NUMBER 21 33 creased salinities of 10%o, as in estuaries a n d
brackish lagoons. It is also e u r y t h e r m i c , b u t can- not survive in waters with t e m p e r a t u r e s below
10° C. T h e r e is a great variability in its mor- phology (i.e., leaf size a n d shape, p l a n t size, ner- vation) which is p r o b a b l y influenced primarily by its e n v i r o n m e n t . In d e n H a r t o g ' s (1970) view, H. ovalis represents a "collective species" a n d only H. ovata ( = H. minor) h a d been separated from it
(Ostenfeld, 1909).
Halophila ovalis is a n a b u n d a n t seagrass in trop- ical a n d w a r m t e m p e r a t e areas, occasionally found in p u r e stands or mixed with Thalassia hemprichii, Halodule uninervis, H. pinifolia, Cymodocea rotundata, or Enhalus acoroides. O n a s a n d - b a r on t h e northwestern e n d of Sumilon Island, Philip- pines, it was observed t h a t Halophila ovalis a p - peared as a pioneer species on new sand-coral rubble substrate. T h e plant flowers a n d fruits d u r i n g t h e warmest m o n t h s . Algal epiphytes found on t h e leaves of t h e plant include crustose melobesioids, a few thalli of species of Acrochae- tium, Ceramium, Hypnea, Jania, a n d Polysiphonia.
PHILIPPINE D I S T R I B U T I O N . — L u z o n (La U n i o n , C a t a n d u a n e s , P a n g a s i n a n , Albay, M i n d o r o , M a s b a t e ) ; P a l a w a n (Cuyo Islands, Q u e z o n ) ; V i - sayas (Leyte, Negros, C e b u , P a n a y , Sumilon, Si- quijor, Bohol, S a m a r ) ; M i n d a n a o (Davao, Z a m - boanga, Bancoran Island).
RANGE.—Halophila ovalis is widely distributed in t h e Indo-West Pacific. It occurs in Egypt, S u d a n , K e n y a , T a n z a n i a , M o z a m b i q u e , South Africa, M a d a g a s c a r , Seychelles, M a u r i t i u s , Israel, Saudi Arabia, Yemen, K u w a i t , Iran, B a h r a i n Islands, Sri L a n k a , B u r m a , A n d a m a n Islands, T h a i l a n d , V i e t n a m , H o n g K o n g , C h i n a , R y u k y u Islands, Malaysia, Indonesia, P a p u a New Guinea, Western Australia, S o u t h Australia, T a s m a n i a , New South Wales, Lord H o w e Island, Queens- land, N o r t h e r n Territory, Caroline Islands, H a - waiian Islands, S a m o a Islands, T o n g a Islands, Fiji Islands, N e w C a l e d o n i a , a n d India.
Halophila spinulosa (R. Brown) Ascherson
FIGURES 23A,B, 24 Caulinia spinulosa R. Brown, 1810:339.
Halophila spinulosa (R. Brown) Ascherson, 1875:368.—Mer- rill, 1915:2; 1925:26.—Pascasio and Santos, 1930:4.—Do- mantay, 1962:275.—Mendoza and del Rosario, 1967:
22.—den Hartog, 1970:263.
CHARACTERISTICS.—Plants small, with long a n d narrow rhizomes, not m o r e t h a n 1 m m in d i a m - eter; internodes 13-35 m m long; usually o n e root per node; roots 5 - 1 0 cm long, beset with filiform hairs. Erect shoots pedicellate with 2 basal, ovate scales with serrate apex. Sessile leaf blades u p to 22 pairs, distichously a r r a n g e d on erect shoot;
blades oblong-linear, semi-amplexicaulous, 20 m m long, 3.5 m m wide; margins serrate; basal portion of one side of b l a d e folded; a few incon- spicuous cross-veins present a n d connected to t h e i n t r a m a r g i n a l nerve; t i p of conspicuous m i d r i b j o i n i n g i n t r a m a r g i n a l nerve.
NATURAL HISTORY.—Halophila spinulosa occurs on sand, m u d , or coral r u b b l e in t h e sublittoral zone. T h e plant m a y grow together with Thalassia hemprichii, Enhalus acoroides, Halophila ovalis, a n d Halodule pinifolia. Flowering H. spinulosa has been observed d u r i n g M a r c h , October, November, a n d December. Fruits were observed in October. T h e plant c a n tolerate brackish water conditions (den H a r t o g , 1970). T h e few specimens of//, spinulosa examined did not reveal a n y epiphytes.
PHILIPPINE D I S T R I B U T I O N . — L u z o n ( M a n i l a Bay, M i n d o r o , C a m a r i n e s ) ; M i n d a n a o (Davao).
RANGE.—Halophila spinulosa occurs in Malaysia, Singapore, Indonesia, Western Australia, P a p u a New Guinea, a n d Q u e e n s l a n d .
Thalassia hemprichii (Ehrenberg) Ascherson
FIGURES 25A,B, 26
Schizotheca hemprichii Ehrenberg, 1836:429.
Thalassia hemprichii (Ehrenberg) Ascherson, 1871c:242.—
Merrill, 1912:71; 1925:27.—Pascasio and Santos, 1930:1, figs. 1-52, plates 1-5.—Domantay, 1962:276.—Mendoza and del Rosario, 1967:50.—den Hartog, 1970:232, fig.
61.—Cordero, 1981:323, fig. 3.
CHARACTERISTICS.—Plants of m o d e r a t e size, consisting of a robust rhizome, 2 - 4 m m in d i a m - eter; internodes 3 - 6 m m long; roots clothed w i t h dense filiform laterals, o n e p e r n o d e if present.
Erect shoot short, with 2 - 6 leaves enveloped by
34 SMITHSONIAN CONTRIBUTIONS TO THE MARINE SCIENCES
FIGURE 23.—Halophila spinulosa: A, habit of plant, X 0.95; B, portion of erect shoot with two amplexicaulous leaves, showing folded basal end, X 3.1.
NUMBER 21 35
FIGURE 24.—Halophila spinulosa: top, world distribution; bottom, Philippine distribu- tion.
36 SMITHSONIAN CONTRIBUTIONS TO THE MARINE SCIENCES
FIGURE 25.— Thalassia hemprichii: A, habit of plant, X 1.0; B, upper portion of leaf showing perpendicular nerves connected by cross veins, X 4.0.
3-8 cm-long sheaths; erect shoots sparsely distrib- uted along rhizome. Leaf blades linear and dis- tinctly falcate (particularly in less grazed areas), 4-10 mm wide, 7-40 cm long (commonly less
than 25 cm long), apices rounded, occasionally uneven, margins entire; 10-16 nerves connected by cross-veins; median nerve often conspicuous.
NATURAL HISTORY.— Thalassia hemprichii is com-
NUMBER 21
37
FIGURE 26.— Thalassia hemprichii: top, world distribution; bottom, Philippine distri- bution.
38 SMITHSONIAN CONTRIBUTIONS TO THE MARINE SCIENCES
m o n on mud-coral-sand or coarse coral-sand sub- strates, in sheltered h a b i t a t s in t h e Philippines.
T h e p l a n t has been observed growing from t h e base a n d t h r o u g h fingers of corals at 6 m deep. T.
hemprichii m a y b e found mixed with Syringodium isoetifolium, Cymodocea serrulata, C. rotundata, Enhalus acoroides, Halophila ovalis, a n d Halodule uninervis. At several sites in Negros, Philippines, T hemprichii a p p e a r e d to be " m o w e d d o w n " in large patches a n d there was evidence of fish grazing on t h e leaves of t h e plant. Algal epiphytes observed on the leaves include species of Centroceras, Cladophora, Erythrotrichia, Fosliella, Giffordia, Herposiphonia, a n d Polysiphonia. Flowering of T hemprichii occurs dur- ing J a n u a r y , February, a n d December in the Philippines.
P H I L I P P I N E D I S T R I B U T I O N . — L u z o n ( M a n i l a
Bay, Pangasinan, C a t a n d u a n e s , Albay, Batangas, C a g a y a n , M i n d o r o , L a U n i o n , Ilocos Norte); Pa- lawan (Quezon); Visayas (Samar, Siquijor, C e b u , Leyte, Bohol, Negros); M i n d a n a o (Surigao, D a - vao, Cavili Island).
R A N G E . — Thalassia hemprichii is c o m m o n in t h e tropical region of t h e I n d i a n O c e a n a n d western part of t h e Pacific. It occurs in S u d a n , Eritrea, Somalia, Kenya, T a n z a n i a , M o z a m b i q u e , Sey- chelles, A l d a b r a Islands, M a d a g a s c a r , Saudi Ara- bia, Yemen, A n d a m a n Islands, T h a i l a n d , Viet- n a m , R y u k y u Islands, Malaysia, Indonesia, P a p u a New Guinea, Caroline Islands, M a r s h a l l Islands, Bismarck Archipelago, N e w Caledonia, a n d Queensland.