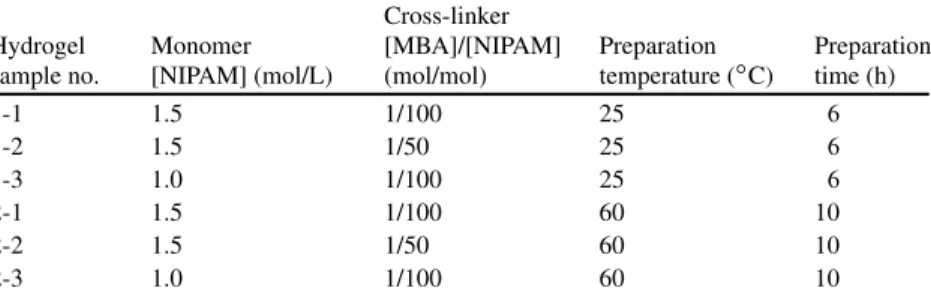
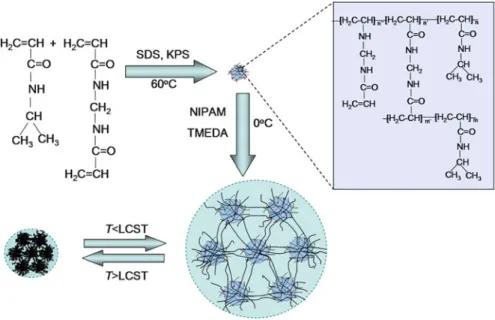
Since in many cases the fluctuations of the ambient temperature can occur naturally and the temperature stimuli can be easily designed and artificially controlled, Part I focuses on the thermo-responsive hydrogel functional materials, in which the structure-function relationship of thermo-responsive hydrogels (Chapter 1), the preparation and properties of monodisperse thermo-responsive microgels (Chapter 2), flow and aggregation characteristics of thermo-responsive microgels during phase transition (Chapter 3), polyphenol-induced phase transition of thermo-responsive hydrogels ( Chapter 4), functional membranes with thermo-responsive hydrogel ports (Chapter 5) and functional microcapsules with thermo-responsive hydrogel shells (Chapter In Part IV, ethanol-responsive hydrogel functional materials, especially smart functional membranes with alcohol-responsive features (Chapter 10 ), are introduced.
Thermo-responsive Hydrogel Functional Materials
Structure-Function Relationship of Thermo-responsive Hydrogels
- Introduction
- Effect of Internal Microstructure on the Equilibrium Thermo-responsive Phase Transition
- Effect of Internal Microstructure on the Dynamic Thermo-responsive Phase Transition
- Effect of Internal Microstructure
The situation for a PNIPAM hydrogel with a heterogeneous internal microstructure, viz. with many microgel-like free ends is very similar to that of comb-grafted PNIPAM hydrogels [5, 6]. The internal microstructures, equilibrium and dynamic phase transitions of MCG P(NIPAM-co-AAc) hydrogels are studied systematically [9].
Effect of Internal Microstructure on the Mechanical Strength of Thermo-responsive Hydrogels
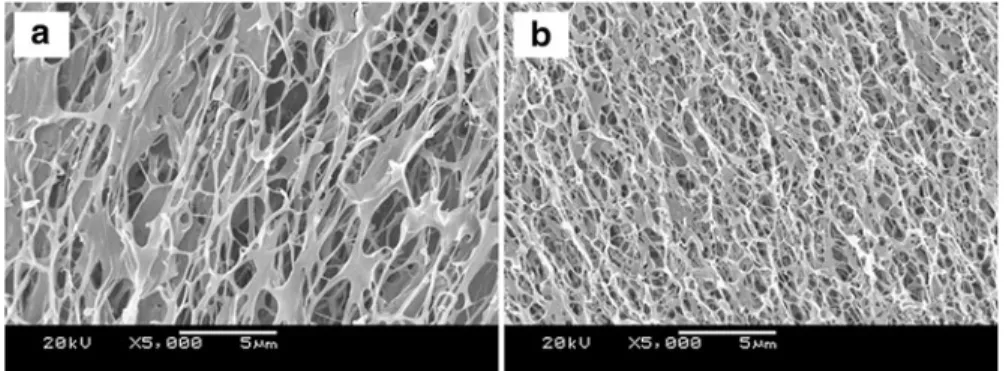
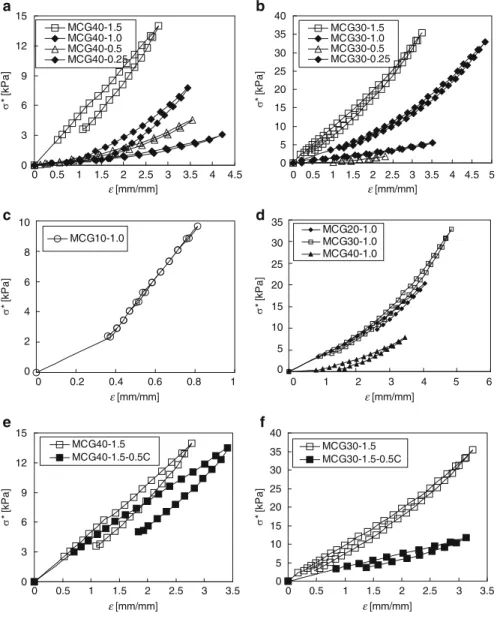
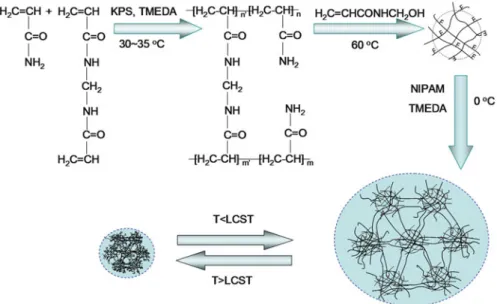
The third kind of MCG hydrogels using active PNIPAM microgels as cross-linkers presents heterogeneous microstructure as cross-linked threads, as shown in Fig. 1.19. A hysteresis circle exists when the third kind of MCG hydrogels recovers after being stretched, as shown in Fig.
Summary
Thermally responsive MCG hydrogels with fast response rate and large volume change with thermal responsiveness and/or improved mechanical strength are achieved by using macromolecular microgels as crosslinkers. So, thermoresponsive hydrogel materials should be designed and fabricated with suitable internal microstructure and molecular structure for different purposes.
These properties of a specific hydrogel are extremely important in choosing which materials are suitable for a given application. For example, for pulse-controlled release systems, a fast response rate to ambient temperature is required; on the other hand, for some separation and extraction systems a large volume change is necessary.
Preparation and Properties of Monodisperse Thermo-responsive Microgels
Introduction
In this chapter, several strategies are introduced, including surfactant-free emulsion polymerization, membrane emulsification and microfluidics to prepare monodisperse thermo-responsive microgels. The type of microgels includes both negative and positive submicron-sized thermo-responsive core-shell hydrogel microspheres and micro-sized thermo-responsive microspheres and microcapsules [41-46].
Submicron-Sized Monodisperse Thermo-responsive Core-Shell Hydrogel Microspheres Fabricated via
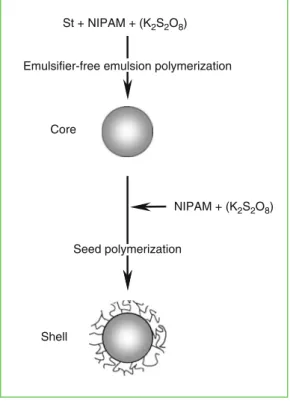
- Preparation of P(NIPAM-co-St) Seeds
- Preparation of Core-Shell Microspheres with PNIPAM Shell Layers
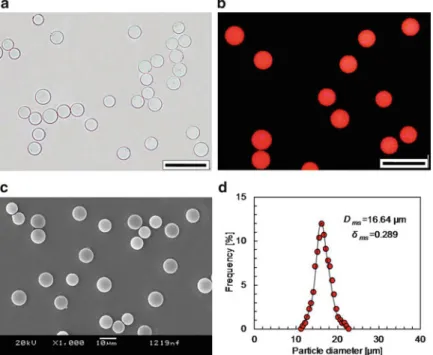
- Monodispersity of Core-Shell Microspheres
- Thermo-responsive Characteristics of the Core-Shell Microspheres with PNIPAM Shell Layers
Therefore, the core-shell microspheres with P(NIPAM-co-St) cores and PNIPAM shell layers are highly monodisperse. Figure 2.3 shows the effect of NIPAM dosage in the preparation of shell layers on the thermo-responsive swelling properties of the core-shell microspheres.
Positively Thermo-responsive Submicron-Sized Monodisperse Core-Shell Hydrogel Microspheres
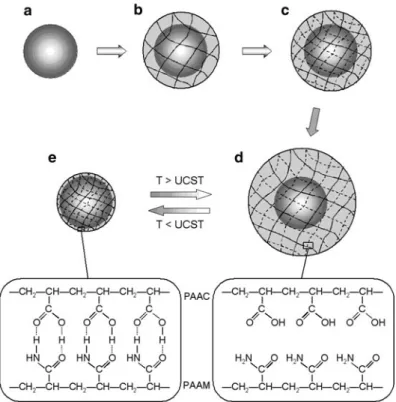
- Preparation of Positively Thermo-responsive
- Morphological Analyses of the Microspheres
- Positively Thermosensitive Swelling Characteristics
The schematic illustration of the fabrication of the positively thermosensitive core-shell hydrogel microsphere is illustrated in Fig. 2.4. A sharp transition of the hydrodynamic diameters occurs from 15 µC to 25 µC, which corresponds to the UCST of PAAM/PAAC-based IPN hydrogels [47].
Monodisperse Thermo-responsive Hydrogel Microspheres and Microcapsules Prepared
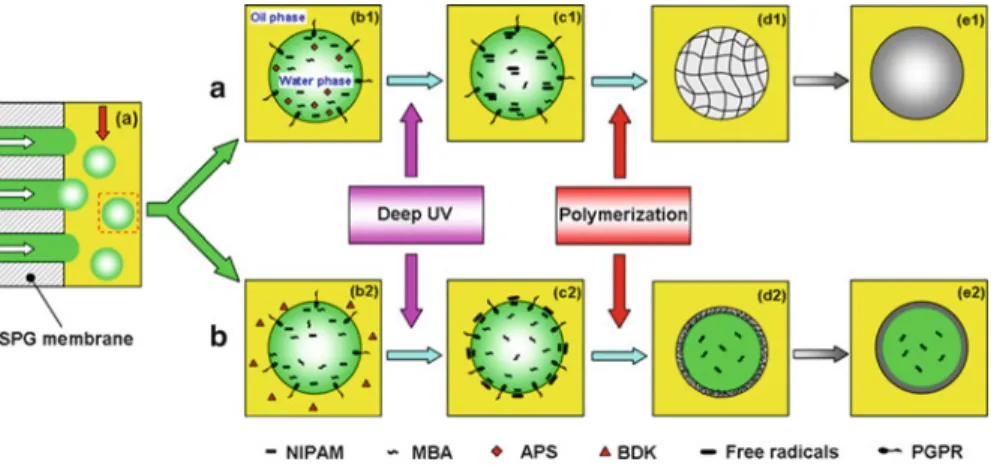
- Strategies for Preparation of Monodisperse PNIPAM Microspheres and Microcapsules via Membrane
- Morphology of Prepared Monodisperse PNIPAM Microspheres
- Morphology of Prepared Monodisperse PNIPAM Microcapsules
- Effect of Freeze-Drying and Rehydrating Treatment on the Thermo-responsive Characteristics of PNIPAM
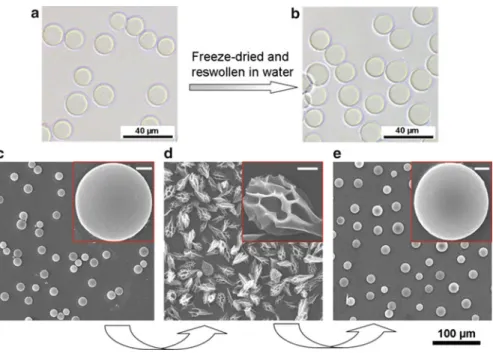
2.8 (a) Optical microgram of PNIPAM microspheres in water, (b) CLSM image of Rd B-stained PNIPAM microspheres, (c) SEM image of air-dried PNIPAM microspheres, and (d) size distributions of the resulting PNIPAM microspheres dispersed in water. The results show that the freeze-drying treatment has almost no effect on the LCST and the equilibrium volume changes of the PNIPAM microspheres.
Monodisperse Thermo-responsive Hydrogel Microspheres and Microcapsules Fabricated
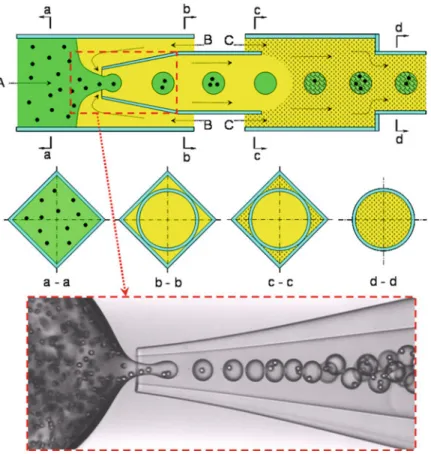
- Microfluidic Fabrication of Monodisperse Thermo-responsive Microgels with Tunable
- Fabrication of Monodisperse Thermo-responsive Microgels in a Microfluidic Chip
- Fabrication of Monodisperse Microspheres with PNIPAM Core and Poly(2-Hydroxyethyl
The schematic illustrations of the structure and the thermo-responsive behavior of the microspheres with PNIPAM core and PHEMA shell are shown in Fig.2.21. Preparation of the core-shell microspheres includes two steps, namely the fabrication of PNIPAM core and PHEMA shell afterwards.
Summary
Vihola H, Laukkanen A, Hirvonen J et al (2002) Binding and release of drugs into and from thermosensitive poly(N-vinylcaprolactam) nanoparticles. Chu LY, Park SH, Yamaguchi T et al (2001) Fabrication of thermo-responsive core-shell microcapsules with a porous membrane and poly(N-isopropylacrylamide) gates. Cheng CJ, Chu LY, Zhang J et al (2008) Effect of freeze-drying and rehydration treatment on the thermo-responsive properties of poly(N-isopropylacrylamide) microspheres.
Flow and Aggregation Characteristics of Thermo-responsive Microgels
During Phase Transition
Introduction
However, if the thermo-responsive microspheres are used for targeting drug delivery systems and other applications, their flow characteristics and kinetic characteristics during the phase transition can directly affect the site-specific targeting performance. Therefore, it is essential and very important to understand the flow properties of thermo-responsive microspheres in microchannels during the phase transition process. Finally, the effects of microchannel surface wetting and roughness on the flow behavior of thermo-responsive microspheres during the phase transition [5] will be systematically discussed.
Flow and Aggregation Characteristics of Thermo-responsive Spheres During
- Preparation of Monodisperse PNIPAM Hydrogel Spheres
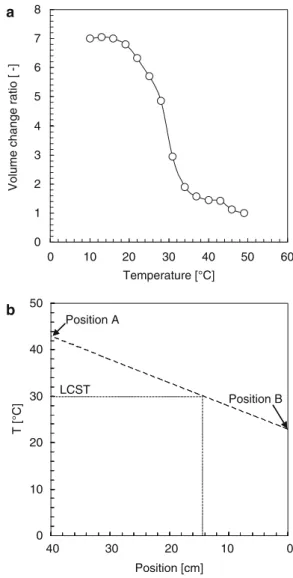
- Thermo-responsive Volume-Phase Transition Characteristics of PNIPAM Hydrogel Spheres
- Flow Characteristics of PNIPAM Hydrogel Spheres During the Phase Transition in a Transparent Glass Pipe
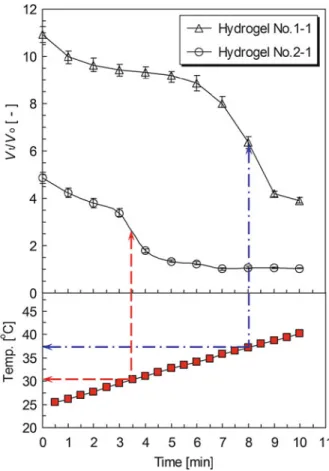
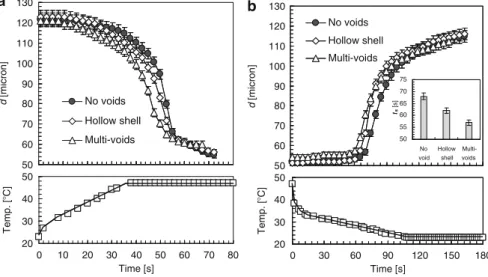
The digital acquisition camera is used to record the flow behaviors of the PNIPAM spheres inside the tube during the phase transition. Many interesting phenomena related to the flow behaviors of PNIPAM hydrogel spheres are found during the phase transition. They finally aggregate together after the phase transition due to the hydrophobic effect of the PNIPAM hydrogel spheres.
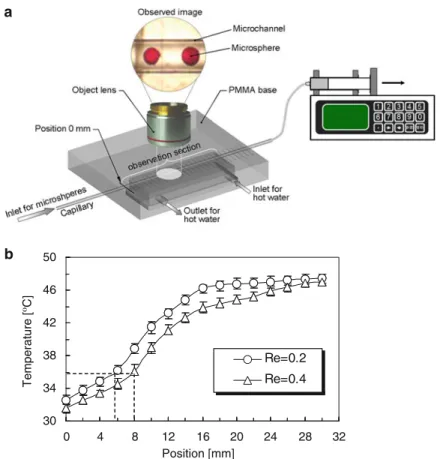
Flow Characteristics of Thermo-responsive Microspheres in Microchannel During the Phase Transition
- Synthesis of Microspheres in a Simple Microfluidic Device
- Flow Characteristics of PNIPAM Microspheres in Horizontal Microchannel at Low Reynolds
- Effect of the Diameter Ratio of PNIPAM Microsphere to Microchannel on the Flow Characteristics
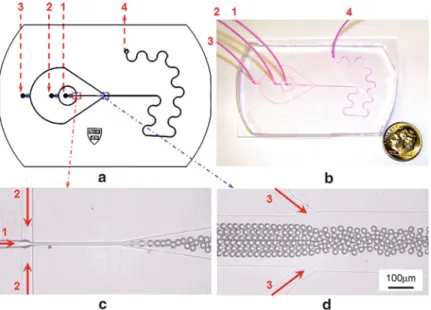
Very interesting phenomena about the flow behavior of PNIPAM microspheres with ds/DmcD0.913 (where ds stands for the diameter of PNIPAM microspheres and Dmc for the inner diameter of the microchannel) are found during the phase transition. Optical micrograph time series showing the movement of a PNIPAM microsphere during the phase transition process. When ten PNIPAM microspheres with ds/DmcD0.596 move along the microchannel (Fig.3.12), they also aggregate together during the phase transition.
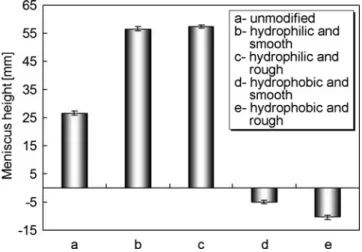
Effects of Microchannel Surface Property on Flow Behaviors of Thermo-responsive Microspheres During
- Modification of Inner Surface of Glass Microchannel
- Characterization of Wettability and Roughness of Modified Glass Microchannels
- Effects of Surface Wettability and Roughness of Microchannel on the Average Velocity
- Effect of Surface Wettability and Roughness
- Effect of Surface Wettability of Microchannel on Flow Characteristics of PNIPAM Microspheres
- Effect of Surface Roughness of Microchannel on Flow Characteristics of PNIPAM Microspheres
- Flow Behaviors of PNIPAM Microspheres in Microchannel with Hydrophobic and Rough
However, not all samples can stop after the phase transition in the microchannel with a hydrophilic and rough inner surface. The results indicate that the rough inner surface of the microchannel may also contribute to the site-specific targeting of PNIPAM microspheres during the phase transition. In the microchannel with hydrophobic and rough inner surface, the microsphere easily stops at a certain position after the phase transition (Fig.3.17).
Summary
Zhou MY, Chu LY, Chen WM et al (2006) Flow and aggregation characteristics of thermoresponsive poly(N-isopropylacrylamide) beads during phase transition. Zhou MY, Xie R, Ju XJ et al (2009) Flow characteristics of thermally responsive microspheres in a microchannel during phase transition. Zhou MY, Xie R, Yu YL et al (2009) Effects of surface wettability and microchannel roughness on phase transition flow behavior of thermoresponsive microspheres.
Polyphenol-Induced Phase Transition of Thermo-responsive Hydrogels
- Introduction
- Phase Transition Behaviors of PNIPAM Microgels Induced by Tannic Acid
- Preparation of Monodisperse PNIPAM Microgels
- Dynamic Isothermal Volume-Phase Transition of PNIPAM Microgels Induced by TA
- Equilibrium Isothermal Volume-Phase Transition of PNIPAM Microgels Induced by TA
- Thermosensitive Phase Transition of PNIPAM Microgels in TA Solutions
- Phase Transition Behaviors of PNIPAM Microgels Induced by Ethyl Gallate
- Preparation of PNIPAM Microspheres and Core-Shell PNIPAM Microcapsules
- Thermo-responsive Phase Transition Behaviors of PNIPAM Microspheres in EG Solution
- The Intact-to-Broken Transformation Behaviors of Core-Shell PNIPAM Microcapsules in Aqueous
- Summary
The higher the TA concentration, the faster the isothermal volume-phase transition of PNIPAM microgels induced by TA. Figure 4.6 further shows the isothermal phase transition rates of PNIPAM microgels as a function of TA concentration in aqueous solutions at 25°C. When the TA concentration is below 105 mol/L, the phase transition rate of PNIPAM microgels increases remarkably.
![Fig. 4.1 Chemical structure of tannic acid (Reproduced with permission from Ref. [3], Copyright (2010), Elsevier)](https://thumb-ap.123doks.com/thumbv2/123dok/10240857.0/108.659.139.522.96.484/fig-chemical-structure-tannic-reproduced-permission-copyright-elsevier.webp)
Functional Membranes with Thermo-responsive Hydrogel Gates
Introduction
Here, the thermoresponsive membranes are fabricated by grafting poly(N-isopropylacrylamide) (PNIPAM) chains onto the substrate membrane via plasma-induced pore-filling graft polymerization and ATRP or by filling PNIPAM into membrane pores via free radical polymerization. The influential factors on the thermo-responsive gate characteristics of the prepared membranes and their applications such as controlled release and affinity adsorption are systematically investigated.
Functional Membranes with Thermo-responsive Hydrogel Gates Fabricated by Plasma-Induced
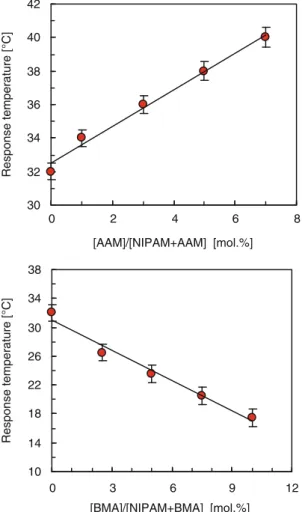
- Regulation of Response Temperature of Thermo-responsive Membranes
- Effect of Grafting Degree on the Thermo-responsive Gating Characteristics
- Gating Characteristics of Thermo-responsive Membranes with Grafted Linear and Cross-linked Hydrogel Gates
- Membranes with Negatively Thermo-responsive Hydrogel Gates
- Composite Thermo-responsive Membrane System
- Thermo-responsive Affinity Membrane
The higher the thermo-responsive gating coefficients, the better the thermo-responsive gating characteristics of the membranes. When the pore filling ratio is less than 44.2%, the pores of PNIPAM-grafted PC membranes show thermo-responsive gating properties due to the conformational change of the grafted PNIPAM in the pores. The degree of grafting of PNIPAM-grafted PE membranes also has an effect on the thermo-responsive models [12].
Functional Membranes with Thermo-responsive Hydrogel Gates Fabricated by Atom-Transfer Radical
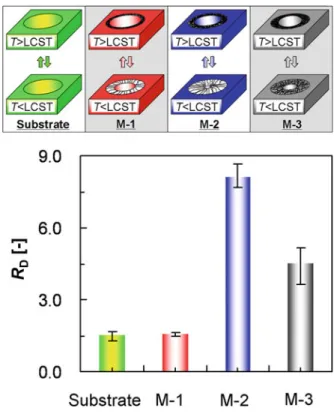
The density of grafted PNIPAM chains on the membrane M-3 is 1.67 times higher than that on membrane M-1, and the graft yield YPNIPAM of membrane M-3 is 2.18 times more than that of membrane M-1 (Table 5.1). Therefore, the length of grafted PNIPAM chains in the pores of membrane M-2 is longer than that in the pores of membrane M-3 (Table 5.1 and Fig. 5.13). The diffusion thermo-responsive coefficient of membrane M-2 (RDD8.1) is greater than that of membrane M-3 (RDD4.4).
Functional Membranes with Thermo-responsive Hydrogel Gates Fabricated by Free-Radical
Therefore, the length of the grafted PNIPAM chains in the M-3 membrane is 1.3 times longer than that in the M-1 membrane. The thermoresponsive diffusion coefficient of the M-3 membrane (RDD4.4) is approximately three times higher than that of the M-1 membrane (RDD1.58) (Fig. 5.13). Compared to the M-1 membrane, the larger RD value of the M-3 membrane resulted from both the higher density and longer length of the grafted PNIPAM chains.
Summary
Yang M, Chu LY, Li Y et al (2006) Thermo-responsive gate properties of poly(N-isopropylacrylamide) grafted membranes. Chu LY, Zhu JH, Chen WM et al (2003) Effect of graft yield on thermoresponsive permeability through plasma-grafted poly(N-isopropylacrylamide) gated porous membranes. Chen YC, Xie R, Yang M et al (2009) Sealing characteristics of linear and crosslinked grafted poly(N-isopropylacrylamide) gated thermoresponsive membranes.
Functional Microcapsules
- Introduction
- Functional Microcapsules with Grafted Thermo-responsive Hydrogel Chains
- Functional Microcapsules with Thermo-responsive Microgels in the Membranes as Gates
- Summary
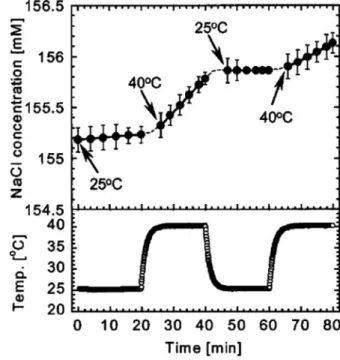
Figure 6.1 shows the thermo-responsive release of NaCl from PNIPAM-grafted microcapsules with an average diameter of approx. 4 m. The thermoresponsive permeability coefficient of NaCl through the PNIPAM-grafted microcapsules with different grafting time is shown in Fig. 6.3. Therefore, the result in fig. 6.3 the effect of the grafting yield on the thermoresponsive permeability coefficient of the PNIPAM-grafted microcapsules.
Preparation and Properties of Monodisperse pH-Responsive Microgels
- Introduction
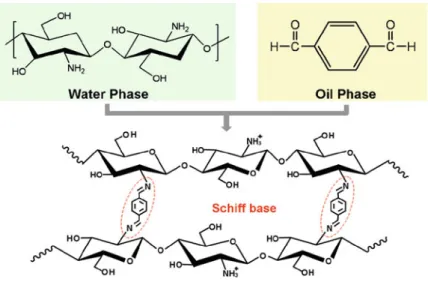
- Monodisperse pH-Responsive Chitosan Microgels
- Monodisperse Cationic pH-Responsive Microgels
- Monodisperse Cationic pH-Responsive Hydrogel Capsules
- Summary
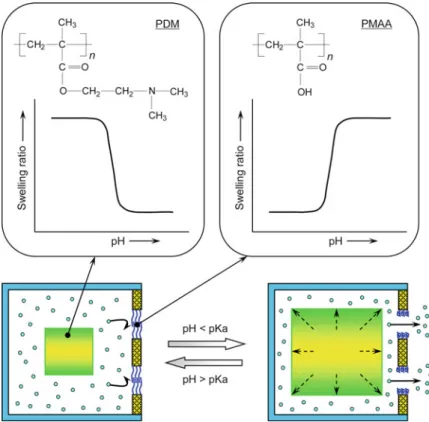
Monodisperse cationic pH-responsive PDM microgels are successfully prepared by dispersion polymerization in an ethanol/water mixture using poly(vinylpyrrolidone) (PVP) as the steric stabilizer and N,N0-methylenebisacrylamide (MBA) as the crosslinker [24]. Figure 7.12 shows the diameters and pH-responsive swelling ratios (IpH/I7.4) of PDM microcapsules prepared at different pH (1# and 2#) [26]. Monodisperse cationic pH-responsive PDM microcapsules can be prepared using O/W/O emulsions as templates via UV-initiated polymerization based on a double initiation system.
- Introduction
- pH-Responsive Gating Membrane System with Pumping Effect for Improved Controlled ReleaseEffect for Improved Controlled Release
- pH-Responsive Microcapsules for Burst Release of Hydrophobic Drugsof Hydrophobic Drugs
- Monodisperse Core/Shell Microcapsules for pH-Responsive Controlled Releasefor pH-Responsive Controlled Release
The pH-responsive pore size changes of the PMAA-g-PVDF membrane are evaluated by filtration experiments with different pH buffer solutions (pH ranges from 2 to 7) [15]. The release rate of a pH-responsive composite membrane system is experimentally investigated using vitamin B12 (VB12) as a model drug [15]. Due to the dual function of the grafted PMAA gate in the porous membrane and the cross-linked PDM hydrogel in the reservoir, the CF value is higher than that of the two systems with a pH-responsive transition.