Oxidation in Biological Systems
Oxidation in Chemical Synthesis
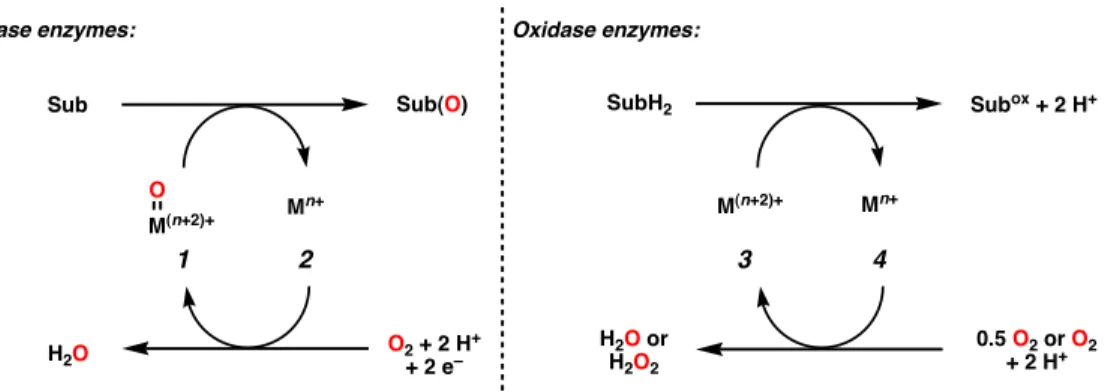
Fundamental reactions of this type include alcohol oxidations and alkane dehydrogenations, both ubiquitous in nature and in chemical synthesis. Although common, asymmetric catalytic variants of this class of reactions have been relatively less investigated compared to the oxygenase mimics.
Notes and References
Introduction
Background
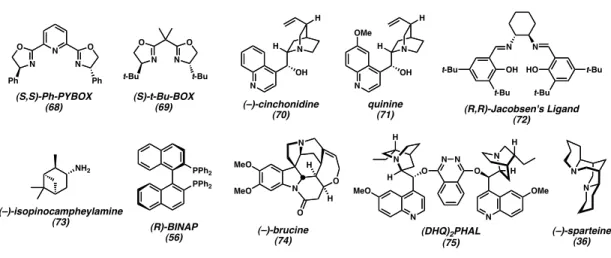
Nitroxyl Radicals for the Oxidative Kinetic Resolution of
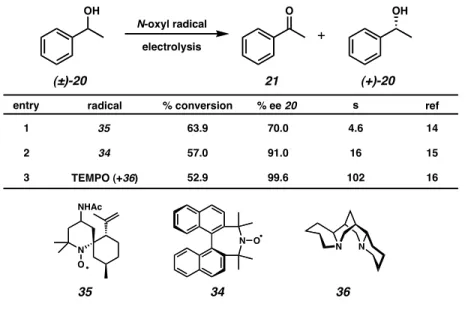
Rychnovsky reported the oxidative kinetic resolution of secondary alcohols using chiral nitroxyl radical 34 (Scheme 2.2.1).12 Sodium hypochlorite acts as an oxidizing agent for nitroxyl radical. Chiral nitroxyl radical 35 resolved sec-phenethyl alcohol (20) with some selectivity,14 while radical 34 (the same as used by Rychnovsky) was significant.
Transition Metal Approaches for the Oxidative Kinetic
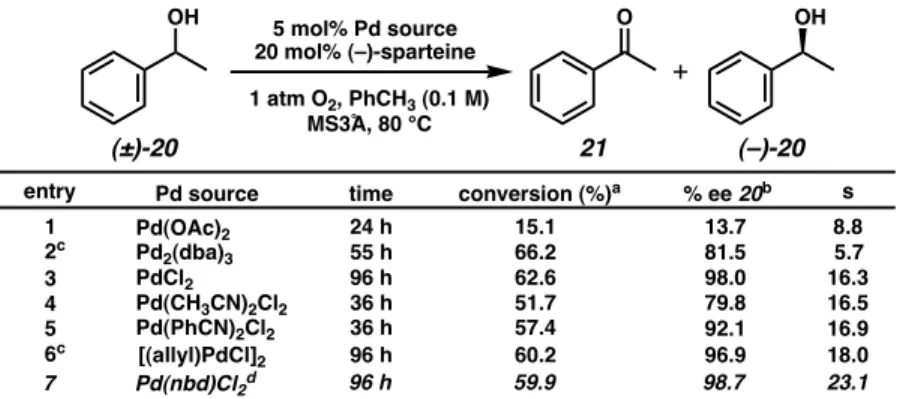
Noyori and Uemura have since developed highly enantioselective variants of the ruthenium-catalyzed kinetic resolution of secondary alcohols. Not only would this system serve as the basis for the development of an oxidative kinetic resolution of.
Reaction Development
- Investigations of a Palladium/Aryl Halide System
- Oxidative Kinetic Resolution of Alcohols with a
- Scale up and Recycling
- Desymmetrization of Meso Diols
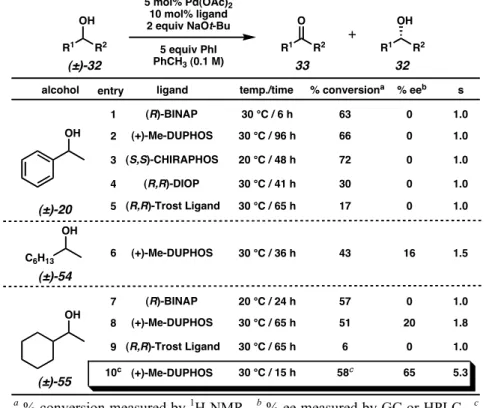
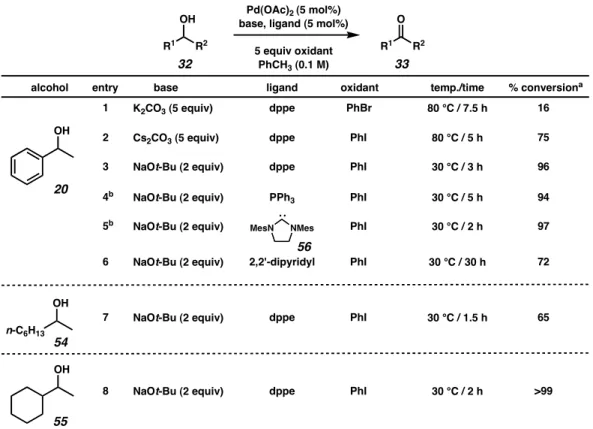
Although sec-phenethyl alcohol (20) was not solvated by any of the palladium–ligand complexes, aliphatic alcohols exhibited modest levels of enantiodifferentiation. Re-examination of the mechanism proposed by Uemura (Scheme 2.3.3) proved critical for the optimization of this reaction.
Further Developments
Improved Reaction Conditions
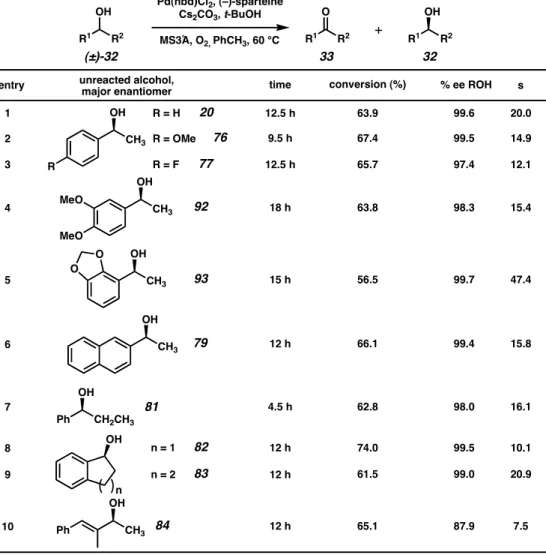
Based on these results, it was predicted that the presence of a stoichiometric base could affect the oxidative kinetic resolution. Hoping to further understand the effects of individual reaction parameters, Jeffrey Bagdanoff conducted a more thorough review of the various conditions for oxidative kinetic resolution.
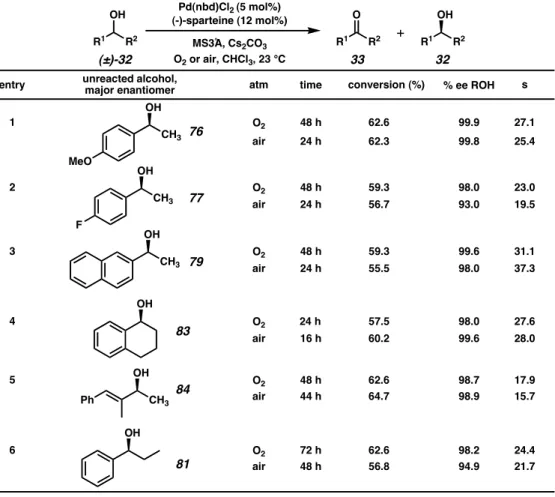
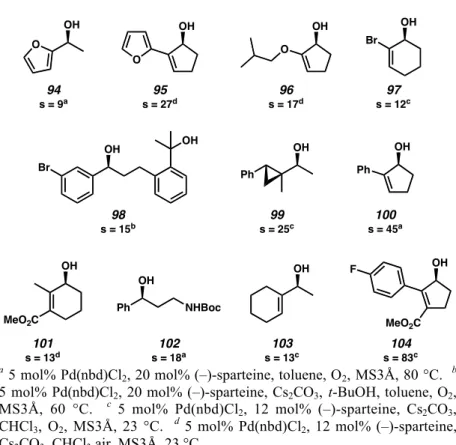
Expanding the Substrate Scope toward Total Synthesis
The palladium-catalyzed oxidative kinetic resolution has also been used in the context of the total synthesis of bioactive natural products (Scheme 2.4.1). Yeeman Ramtohul, a former postdoctoral researcher in the Stoltz lab, applied the resolution to secondary alcohol 105, which we expected to be a viable intermediate in the enantioselective synthesis of aurantioclavine (106).39 Michael Meyer, a graduate student in the Stoltz Laboratory, has also performed a resolution of cyclopentenol 107 as a method to access both enantiomers of an important intermediate in the work towards the total synthesis of bielschowskysine (109).40.
Mechanistic Studies
Coordination Studies on Palladium-Sparteine Systems
The phenyl (RL) is in the open region above the square face of the metal complex (111, side view), and the benzylic C-H bond faces the metal plane parallel to the palladium-chloride bond. Importantly, the palladium-chloride bond is slightly distorted from the square plane, implying how the chloride anion moves away from the metal center revealing a site for β-hydride elimination. Due to steric interactions with the sparteine ligand, alkoxide 112 cannot assume the necessary orientation to undergo β-hydride elimination; it therefore protonates and dissociates from the complex.
Computational Studies
Meanwhile, alkoxide 113 can undergo partial chloride dissociation and β-hydride elimination from the four-coordinate metal center with an axially positioned chloride.42 Since alkoxide 113 leads to ketone production and alkoxide 112 leads to alcohol dissociation, overall resolution occurs.
Kinetic Studies of Palladium(II) Oxidative Systems
Stahl also investigated the catalyst conversion steps that follow β-hydride elimination.49 Using the bathocuproine ligand, Stahl was able to oxidize the palladium(0) center to palladium(II) with molecular oxygen. 114 was converted to the diacetate in the presence of acetic acid, demonstrating how the diamine-palladium(0) intermediate species can regenerate the catalytically active diamine-palladium(II) species with molecular oxygen.50 Uemura proposed an alternative pathway where oxygen simply inserts into palladium-hydride bond formed in the β-hydride elimination step, thus avoiding the palladium(0) intermediate altogether.26f.
Conclusion
Experimental Section
Materials and Methods
Preparative Procedures
The mixture was extracted with Et 2 O (2 x 100 mL) and the combined organic phases were washed with brine, dried over MgSO 4 and concentrated to an oil. The mixture was cooled to room temperature, filtered with suction, and the filtrate was concentrated in vacuo. The mixture was concentrated in vacuo and the residue was dissolved in CHCl 3 /EtOH (1:1, 60 mL).
Palladium(II) Oxidation Procedures
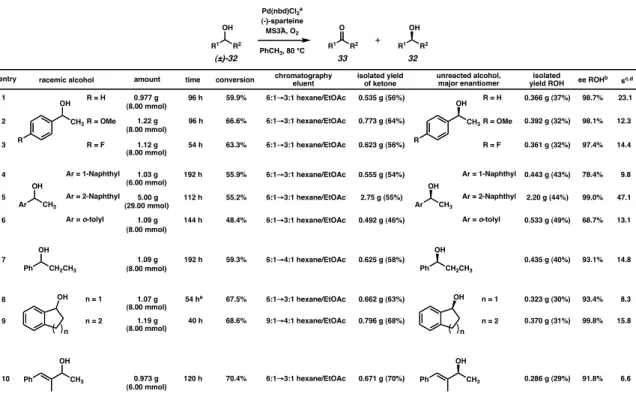
Alcohol (0.50 mmol, 1.0 equiv) was introduced and the reaction was monitored by standard analytical techniques (TLC, GC, 1H NMR and HPLC) for % conversion and enantiomeric excess values. The flask was evacuated under vacuum and filled with O2 (3x, balloon) and the reaction mixture was heated to 80 °C for 10 minutes. Alcohol (8.00 mmol, 1.0 equiv) was introduced and the reaction was monitored by standard analytical techniques (TLC, GC, 1H NMR and HPLC) for % conversion and enantiomeric excess values.
Notes and References
This effect has been attributed to the hydrogen bonding ability of CHCl3. combined with its ability to dissolve chloride anions. The enantioselective synthesis of aurantioclavine has been pursued as part of an approach to the total synthesis of communisin B; see, May, J. California Institute of Technology, Pasadena, CA. 42) Elimination of β-hydride from a five-coordinate palladium center (where the hydride is oriented in an axial position above the metal plane) was considered but found to be significantly higher in energy than the four-coordinate transition states in theoretical calculations.43. California Institute of Technology, Pasadena, CA. 57) Percent conversions were measured by GC integration of alcohol and ketone peaks, correcting for response factors (for conditions, see Table 2.7.4).
Introduction
Background
Based on our successes in the palladium-catalyzed oxidative kinetic resolution of secondary alcohols,2a we speculated that a similar oxidation system could be used for the development of asymmetric intramolecular Wacker reactions. Specifically, the alcohol oxidation system described by Uemura (Pd(OAc)2, pyridine, O2)9 was used as a starting point for the discovery of the kinetic resolution (Pd(nbd)Cl2, (–)-sparteine, O2). Described herein are our attempts to apply the palladium(II) aerobic oxidation system we have studied to intramolecular Wacker cyclizations to form heterocycles.
The Development of Palladium-Catalyzed Aerobic
Oxidative Cyclizations with Molecular Oxygen
Initial Experiments
In addition, enantioselectivity increased measurably when 10 mol% AgSbF6 was added to the reaction mixture.12 Although selectivities were modest, these encouraging results demonstrated the feasibility of extending the simple palladium/ligand/oxygen/toluene system to enantioselective variants.
Reaction Development
Importantly, highly enantioselective variants of these oxidative heterocyclizations can be realized under similar systems (Scheme 3.3.4).4 Again, using (–)-sparteine as the chiral ligand, the cyclization of phenols 24 and 170 proceeded with high enantioinduction in dihydro-funzoaf. 2 5 (81% ee) and 164 (90% ee), respectively. These results represented the first examples of palladium-catalyzed asymmetric heterocyclizations using molecular oxygen as the sole stoichiometric oxidant. This was not only applicable in alcohol oxidation chemistry, but was now also possible in Wacker oxidative cyclizations.
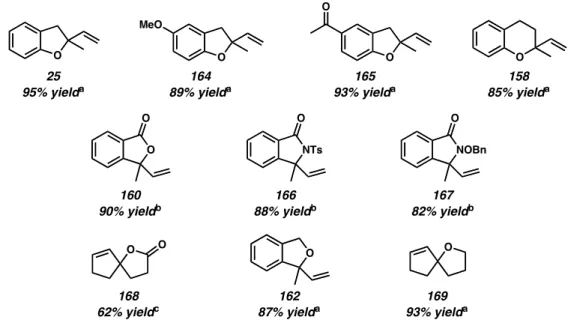
The Formal Total Synthesis of Cephalotaxine
- Introduction and Background
- Retrosynthetic Plan
- Formal Total Synthesis of (±)-Cephalotaxine
- A Second Formal Total Synthesis of
Although the synthesis of cephalotaxin has been extensively studied, we envisioned that the synthesis of this molecule would serve to illustrate the utility of palladium(II) oxidative chemistry that we have studied thus far. Therefore, in case of development of asymmetric heterocyclization, it can be easily used for the enantioselective total synthesis of (–)-cephalotaxine. In relation to the first of these questions, we investigated heterocyclizations that can proceed under catalytic conditions and can be used for the synthesis of cephalotaxin (Scheme 3.4.6).
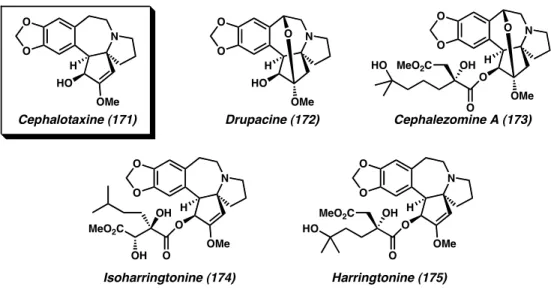
A Proposed Total Synthesis of Drupacine
We hypothesized that the known racemization of this compound could be used as a key transformation in the enantioselective total synthesis of (–)-drupacin (172), another member of the Cephalotaxus alkaloids.30. The diketone moiety in the diastereomer 215 is positioned in close proximity to the benzyl alcohol, allowing acetal formation under acidic conditions. This dynamic diastereomeric resolution is expected to eventually direct all material to acetal 212 .
Conclusion
This synthesis would be highly illustrative of the synthetic utility of the aerobic palladium(II) transformations we have developed thus far. We have also begun to apply some of these reactions toward the synthesis of the Cephalotaxus alkaloids, completing two formal syntheses of (±)-cephalotaxine. Hoping to further develop palladium(II)-catalyzed dehyrogenative reactions, there were two key directions that we believed needed extensive investigation.
Experimental Section
Materials and Methods
Preparative Procedures
The mixture was extracted with CH 2 Cl 2 (4 x 75 mL) and the combined organic phases were dried over Na 2 SO 4 and concentrated to an oil. The mixture was cooled to room temperature and the volatile solvent was removed by rotary evaporation. The mixture was allowed to warm to room temperature and stirred until a white precipitate formed.
Notes and References
This contributed to the idea that the palladium counterion would have an effect on the selectivity and reactivity of the reaction, as we saw in the kinetic resolution chemistry. The elucidation of the mechanism in similar reactions will be discussed as part of Chapter 4 of this thesis. However, for the purposes of accessing large amounts of material, the palladium/DMSO conditions were more effective.
Introduction
Carbon-Carbon Bond Forming Reactions via
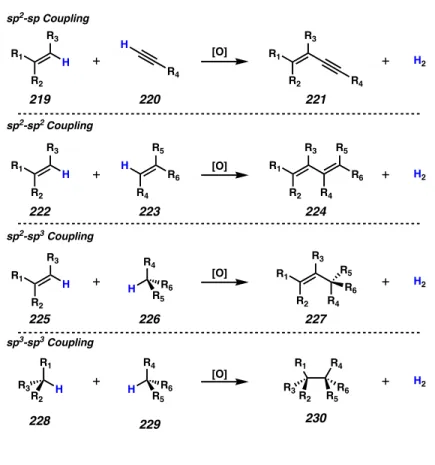
A number of oxidative transformations that can be imagined in such a process are depicted in figure 4.1.1. These transformations result in the functionalization of two C-H bonds and the formation of a new C-C bond. Carbons from any hybridization (sp, sp2, or sp3) can be viewed as coupling partners for these dehydrogenative bond-forming reactions.
Background
Palladium(II) Oxidative Arene-Olefin Couplings
Recently, Jacobs has described a similar system in the oxidative coupling of benzene derivatives and activated esters (Scheme 4.2.1).12 In the presence of Pd(OAc)2 and a cocatalytic amount of benzoic acid below approx. 8 atm O2, benzene derivatives could be oxidatively coupled to give styrenyl compds. Palladium-mediated oxidative coupling reactions involving the indole nucleus have been extensively studied by Itahara and co-workers.14 However, catalytic examples were consistently plagued by low yields (Scheme 4.2.3). Fujiwara recently described a simple example of a catalytic intermolecular oxidative coupling using the indole core.15 Under Pd(OAc)2 and a benzoquinone/TBHP reoxidation system, indole (245) was coupled to methyl acrylate to give 246 in 52% yield.
Synthetic Importance of Annulated Indoles
In 1978, Trost reported a palladium-mediated cyclization and subsequent reduction of indole 252 to form (+)-ibogamine.19,20 Fifteen years later, Williams described the total synthesis of paraherquamide B using a similar palladium-promoted cyclization ( II). reduction sequence of indole 254 as key transformations.21 Recently, Corey reported the oxidative cyclization of indole 257 using palladium(II) in syntheses of members of the austamide class of natural products.22,23 Although all cyclizations were efficient in a synthetic context, all required stoichiometric amounts of palladium (II) salts. In comparison, the palladium/pyridine/O2 catalytic system we studied was found to be quite efficient at a.
The Synthesis of Annulated Indoles via Palladium-Catalyzed
- Reaction Development
- Susceptibility of Annulated Indoles to Oxygen
- Substrate Scope
- Mechanistic Insights
- Methodology Comparisons
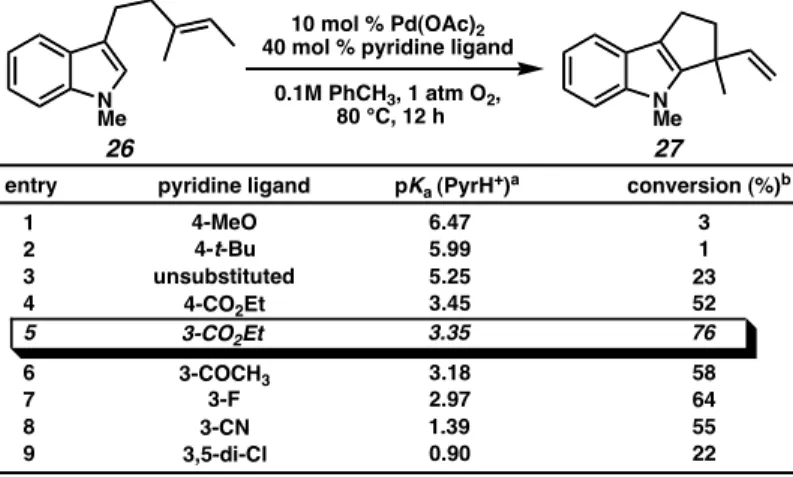
Canceled indole 27 was subjected to the reaction conditions for 24 hours and decomposition was monitored by GC analysis against an internal standard (tridecane).34 A slight increase in product stability was observed with t-amyl alcohol versus toluene, consistent with our observations of the cyclization reaction. Addition of the catalyst (10 mol% Pd(OAc)2, 40 mol% ethyl nicotinate) had a remarkable impact, limiting product decomposition to approximately 30% within 24 hours. The availability of only one β-hydrogen and the general assumption of both antinucleophilic attack and elimination of syn-β-hydride explain the expected stereochemistry of the product indole.
The Synthesis of Benzofurans and Dihydrobenzofurans via
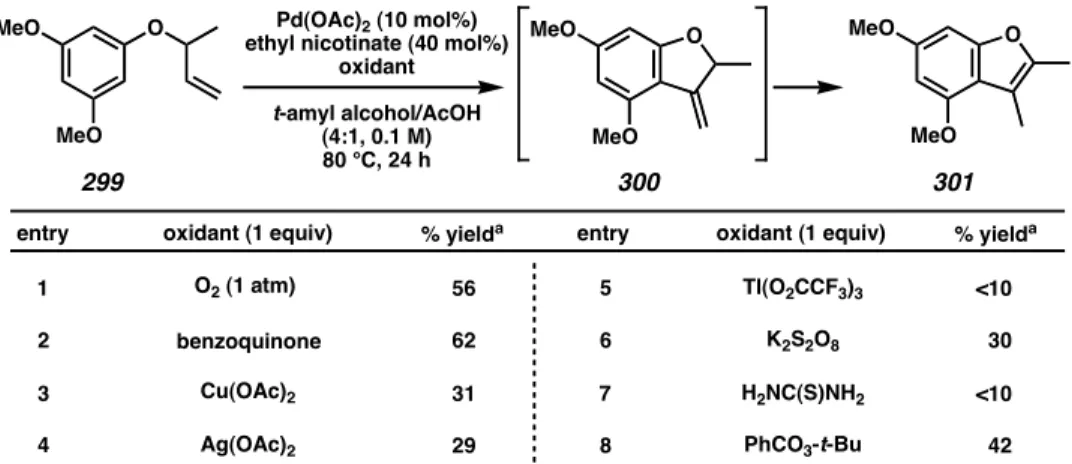
This likely reflects a balance between sufficient binding of the palladium center for Pd(0) reoxidation and suppression of competing binding caused by the presence of excess ligand. As shown in Table 4.4.3, this process works for a variety of ali-aryl ethers with different substitution patterns, all resulting in good yields. Analogous to the indole annulation studies (video above), aryl allylic ether 344 was subjected to cyclization conditions as a mechanistic probe (Scheme 4.4.1).
Future Directions
In the event, the dihydrobenzofuran product (346) was isolated as a single diastereomer.43 This experiment strongly suggests that the palladium pathway (a net functionalization of the C–H bond) is operative, which correlates with the result from the indole study. We have demonstrated that the Pd/pyridine system can be easily modified into enantioselective versions at other reaction multiplexes.1,2 Perhaps the same concept can be applied to the carbocyclization chemistry described here (e.g. to place quaternary centers in enantioselective.46 The carbocyclization studies described above have clear and immediate implications for total synthesis and methodology.
Conclusion
This electronic tuning has led to the discovery of unique reactivities that were not available under the original conditions. This is directly analogous to the corresponding intramolecular Heck reaction, but does not include the prior halogenation required for the palladium (0) process. In addition, the oxidative carbocyclization can be considered orthogonal to the Heck reaction as the electron-rich aromatic systems used in this study can be used directly.
Experimental Section for the Oxidative Annulation of
Materials and Methods
Preparative Procedures
The organic layers were combined, washed with brine, dried over MgSO 4 and concentrated in vacuo. The organic layers were combined, washed with brine, dried over Na 2 SO 4 and concentrated in vacuo. The organic layers were combined, washed with brine, dried over MgSO 4 and concentrated in vacuo.
Palladium-Catalyzed Indole Annulations
Independent Synthesis
Materials and Methods
Preparative Procedures
Palladium-Catalyzed Benzofuran and
Notes and References
Introduction
Heterocyclizations of Carboxylic Acids
Heterocyclizations of Amines
Studies of the Cyclizations of Tosylamides
Studies of the Cyclizations of Tosylanilines
Probing the Mechanism
Monodentate and Bidentate Ligand Effects
Ligand:Palladium Ratio Studies
Stoichiometric Palladium Cyclizations
Independent Synthesis of Tosylindoline 412
Palladium Hydride Mediated Olefin Isomerizations
Distinguishing between a Wacker Mechanism and
A Proposed Stereochemical Model for the Observed
Further Developments
Conclusion
Experimental Section
Materials and Methods
Preparative Procedures
- Synthesis of Ligands
- Synthesis of Carboxylic Acid Substrates
- Synthesis of Amine Substrates
Palladium(II) Heterocyclizations
Independent Syntheses of Heterocycles
Notes and References