I hereby declare that the work contained herein is solely the result of investigations carried out by me in the Department of Chemical Engineering, Indian Institute of Technology Guwahati, Guwahati, Assam, India under the supervision of Dr. Prasanna Venkatesh Rajaraman Assistant Professor Department of Chemical Engineering Indian Institute of Technology Guwahati Guwahati-781039, Assam, India.
ACKNOWLEDGEMENT
I thank them from the bottom of my heart for always believing in me and inspiring me in the early stages of my life. A very special thanks to Udayan Banik for endless support in fixing, suggestions on my work and every possible way.
ABSTRACT
From the parameters obtained, dominance of Co (III) compound formation is observed for both systems. The results confirm the formation and dominance of Co-oxalate complexes for H2O2+oxalic acid system.
NOMENCLATURE
English Symbols
Greek Symbols
110 4.3 Control of galvanic corrosion with oxalic acid and imidazole for chemical mechanical planarization of cobalt-copper interface. 1 Calculated corrosion potential (Ecorr), corrosion current density (Icorr) and galvanic corrosion current (Igc) from potentiodynamic polarization plots.
THIS PAGE IS LEFT BLANK INTENTIONALLY
INTRODUCTION 1 Overview
- Importance of CMP
- Advantages and Disadvantages of CMP
- Advantages
- Disadvantages
- Applications
- Basics and principle of CMP
- Input Variables (Li, 2007)
- Output Variables(Li, 2007)
- Challenges in CMP
- Evolution of CMP .1 Interconnect Materials
- Introduction of Ruthenium (Ru) and Cobalt (Co) as barrier for Cu diffusion It is not possible for a material to have all the properties as per mentioned above to act as a
- Galvanic Corrosion
- Electrochemistry
- Open circuit potential (OCP)
- Potentiodynamic polarization (PP)
- Electrochemical Impedance Spectroscopy (EIS)
- Electrical Equivalent Circuit Model (EEC)
- Reaction Mechanism Analysis (RMA)
Transistor device manufacturing is known as the front of the line (FEOL), while the back of the line (BEOL) is the interconnection part (both local and global wiring) that connects different elements in a circuit (Krishnan, Nalaskowski and Cook, 2010). On the other hand, cathodic polarization occurs when electrons associate with the surface of the working electrode (equations 1.7).
LITERATURE REVIEW
- Aluminum (Al), the first-generation interconnect
- Study on Chemical Mechanical Polishing and Galvanic Corrosion of Copper With the upgradation of the devices, Cu (1.67 μΩ) is introduced as an interconnect in place of
- Ammonium hydroxide (NH 4 OH) based slurry
- Nitric Acid based CMP Slurry
- Peroxide based CMP slurries
- Other Slurries
- Study on Chemical Mechanical Polishing of Tantalum and its Nitride (Ta/Ta 2 N/TaN)
- Study on Chemical Mechanical Polishing of Ruthenium
- Galvanic corrosion of Ruthenium and Copper
- Study on Chemical Mechanical Polishing of Cobalt (Co)
- Galvanic Corrosion of Cobalt and Copper
- Dissolution Mechanism of Co by EIS
- Lacunae
- Objectives
Wang et al. (Wang et al., 1998) reported that the dissolution of Al compounds is directly proportional to glycine concentration and slurry pH. Copper removal depends on the deposited texture and impurity content (Feng et al., 2008). Potentiodynamic studies by Eli et.al (Ein-Eli et al., 2003) confirm active dissolution at different concentrations of nitric acid.
It should be noted that aging time plays a key role in slurry composition and removal rate of the metal (Kim et al., 2022). Yadav, Jitendra C. Bisen, et al., 2017) Therefore, the addition of several oxidizing agents and additives was suggested for Ru CMP. In an acidic medium, Ru is oxidized and forms ruthenium dioxide (RuO2), which is further converted to ruthenium tetraoxide (RuO4).(Wei et al., 2007a).
Also, optimized GC concentration together with 1,2,4 triazole in H2O2-based slurry can provide a desired Cu-Ru selectivity. (Wang et al., 2018). Therefore, with an increase in the concentration of the ions, the removal rate increases. (Ziyan Wang et al., 2019) An optimized concentration could also provide a relatively smoother surface.
EXPERIMENTAL MATERIALS AND METHODOLOGY
- Materials
- Chemical Mechanical Polishing Experiments
- Static Etch Experiments
- Electrochemical Experiments
- Methodology
- Chemical Mechanical Polishing Experiments
- Modelling methods
- Electrical Equivalent Circuit (EEC) model
- Reaction Mechanism Analysis (RMA)
- Characterization studies
- X-ray Diffraction (XRD) Measurements
- Fourier Transform Infrared Spectroscopy (FTIR)
- Ultra Violet Visible Spectrophotometer (UV Vis)
- Field Emission Scanning Electron Microscopy (FESEM)
- Contact angle analysis
- X-ray photoelectron spectroscopy (XPS)
- Thermogravimetric Analysis (TGA)
- Investigation of Polishing Characteristics of Interconnect (Cu) – Barrier Metal (Ru) in Potassium Iodate-Based Slurry
- Motivation
- Experimental Conditions
- Results and Discussions
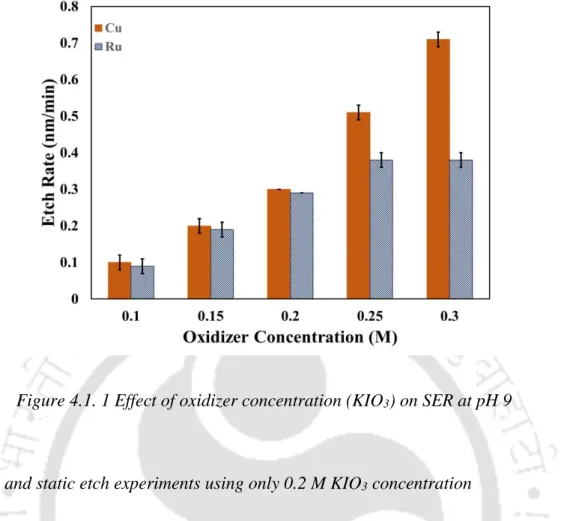
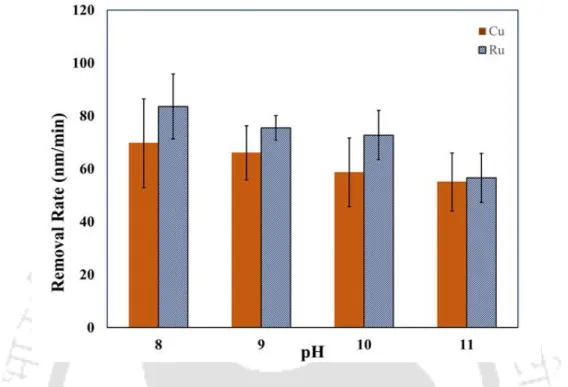
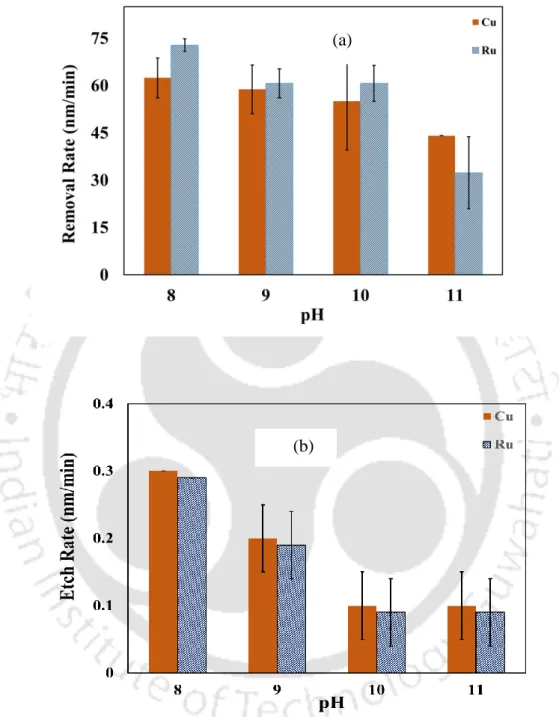
To gain an insight into the chemical dissolution of the metal (Cu, Co and Ru) in the presence of different chemical reagents, etch rate experiments were carried out. The change in the Eoc value indicates the change in the nature of the metal surface. Alumina (Beuhler, India) and titania (TTP, India) abrasives were also tried as part of the experiment.
Activation energy (Ea) of Cu and Ru solution of the proposed solution (0.2 M KIO3) was calculated using the Arrhenius equation (Yadav, Jitendra C Bisen, et al., 2017; Gupta, Manivannan, and Noyel Victoria, 2019) . This explains the reason for lower etch rates of the metals in the KIO3 solution. The further reduction of the dissociated 𝑂2 to 𝑂𝐻− plays the main role in the oxidation of the metal surface.
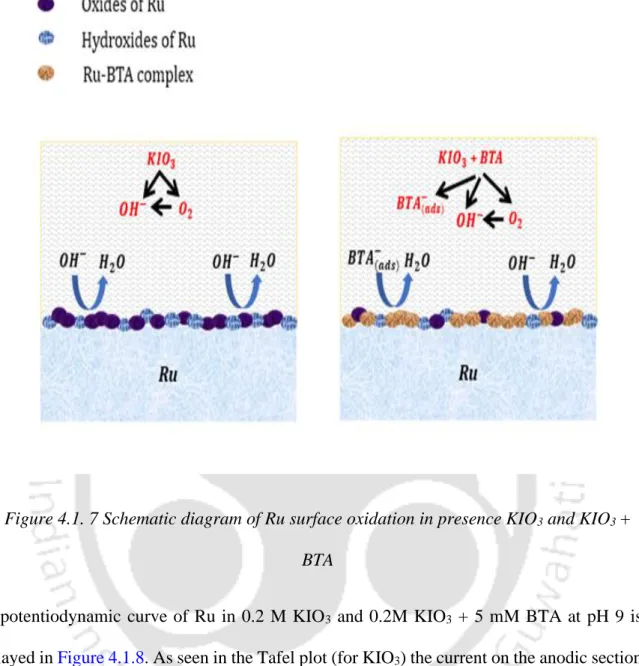
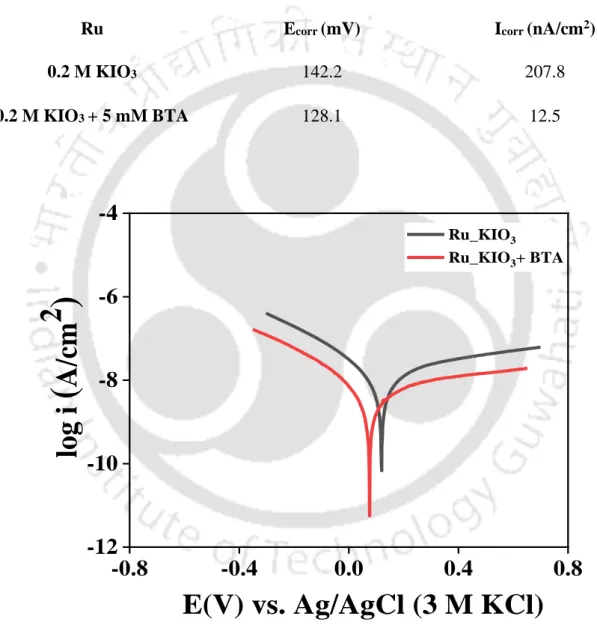
The formation of the Ru hydroxides and oxides in the alkaline region can be aided by the Ru Pourbaix diagram (Han, 2016). In the presence of the inhibitor BTA, the anodic current decreases, indicating BTA adsorption on the metal surface.

Conclusion
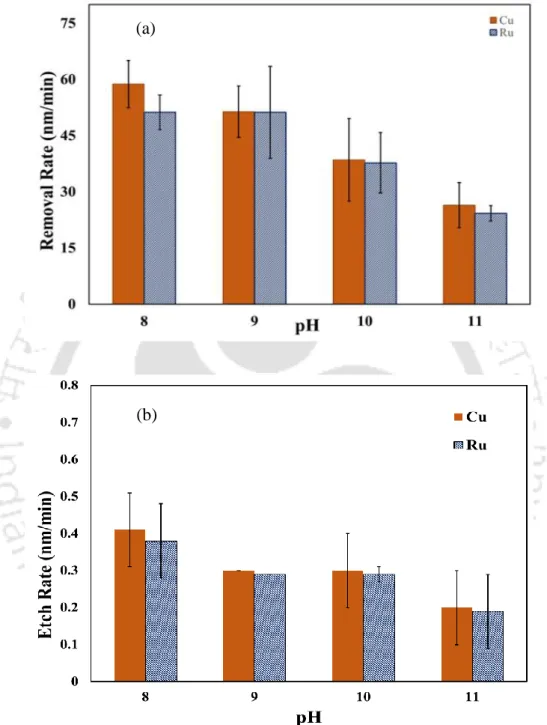
In this work, the role of KIO3 as an oxidant in CMP of Cu-Ru was studied. The study was carried out in the alkaline region, to prevent the appearance of RuO4, which is toxic in nature. This occurs due to the lesser availability of 𝐼𝑂3− ions to oxidize the metal surface beyond pH 7.
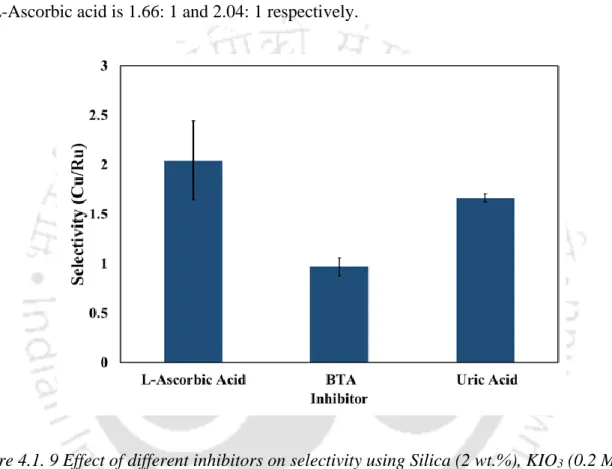
This is due to the occurrence of a chemical reaction on the metal surface at high temperature. The dissolution of metals in the etchant follows an endothermic nature and the measured activation energy Ea was 27.3 KJ/mol for Cu and 25.2 KJ/mol for Ru. However, the desired Cu-Ru selectivity of ~1:1.03 was achieved at pH 9, indicating that a solution containing fumed silica (2 wt%), KIO3 (0.2 M), and BTA (5 mM), presumably suitable for efficient polishing of barrier metal and junctions that may be used in the semiconductor industry.
It was determined that the system does not satisfy Preston's empirical equation with pressure and table speed. It was also observed that the process parameters play a negligible role on the selectivity at the given optimized slurry.
Formulation of sodium hypochlorite-based slurry for copper - cobalt chemical mechanical planarization process
- Motivation
- Experimental Conditions
- Results and Discussion
- CONCLUSION
It should be noted that Cu and the barrier metal, Co CMP, have been mainly incorporated into ULSI devices consisting of CMOS, FET (PMOS and NMOS transistors), etc. Thus, this study mainly focuses on slurry formulation to find the desired ~1:1 selectivity of Cu and Co using sodium hypochlorite as oxidant, fumed silica as abrasive. The static etch rate of Co decreases from 9.54 nm/min to ~ 2 nm/min when the pH of the solution shifts from the acidic to the alkaline regime, as observed in the polishing experiments.
In the same way, the removal rates for Cu show the decreasing trend (~792 nm/min to 66 nm/min) with respect to pH value. As expected, improvement in the polishing rate is observed due to the mechanical abrasion between substrate and abrasive particles. Only a slight decrease in the polishing rate is observed with the addition of BTA for both the metals.
It should be noted that all polishing experiments were performed on Cu and Co coupons in the present study. This indicates the formation of a stable passive layer on both metal surfaces in the alkaline regime, resulting in less dissolution of the metal compared to that in the acidic regime.

Controlling Galvanic Corrosion with Oxalic acid and Imidazole for Chemical Mechanical Planarization of Cobalt-Copper Interface
- Motivation
- Experimental Conditions Electrochemical experiments
- Results and discussion
- Conclusion
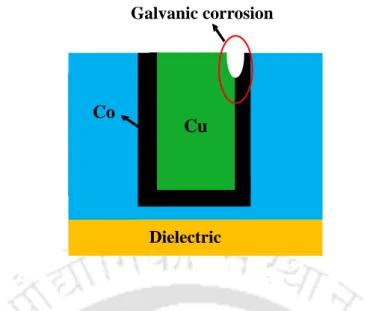
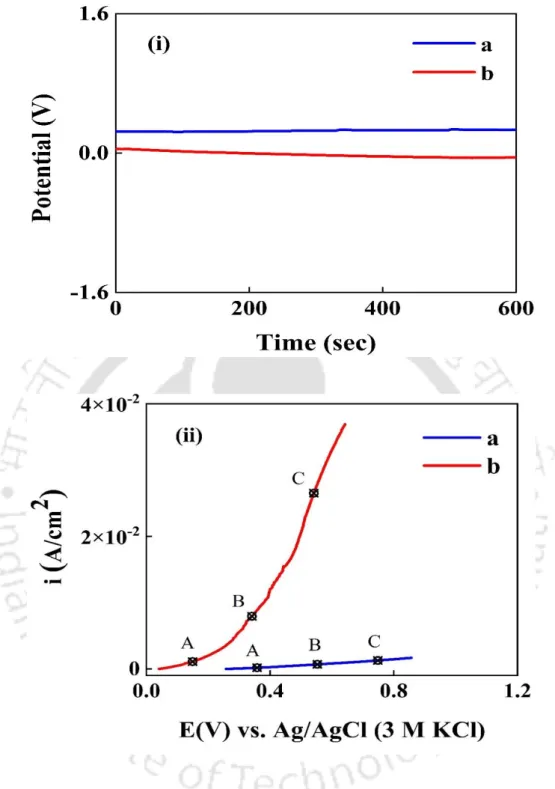
Popuri et.al (R. Popuri et al., 2017) studied the effect of potassium oleate (PO) as a corrosion inhibitor for Co CVD films at neutral pH for both bulk removal (step 1 of the damascene process) and barrier removal/removal of interconnections (step 2 of the damascene process) in H2O2. Therefore, in a Co-Cu galvanic pair, Co will act as the anode while Cu will act as the cathode. (Bilouk et al., 2009b; . Shimizu, Sakoda and Shimogaki, 2013) A schematic of the galvanic corrosion between Cu and Co is shown in Fig. 4.3.1, while the potentiodynamic polarization plot of Co and Cu in DI water at pH 9 is shown in Fig. 4.3.2. The reduced SER values in the alkaline range relative to low pH values are attributed to the formation of a passivation film on the metal surfaces of Co and Cu. (Peethala et al., 2012) This film prevents the metal from dissolving in the alkaline range.
According to the literature (Peethala et al., 2012), a small amount of H2O2 efficiently inhibits the corrosion of the Co-Cu couple. It should be mentioned that the galvanic corrosion current (igc) is calculated from the (E, i) coordinates of the intersection of the Co anodic Table branch and the corresponding Cu cathodic Table branch. (Turk et al., 2015) Keep in mind that the galvanic current density strongly depends on the potential gap of the two metals. Imidazole is a nitrogenous heterocyclic compound of azole group and reported as an effective corrosion inhibitor for various metals such as Cu, Ru, Al. (Gašparac, Martin and Stupnišek-Lisac, 2000; Stupnišek-Lisac, Gazivoda and Madžarac, 2002 ; Lee, 2003; Bui et al., 2012; He et al., 2014; Ko et al., 2021) How polishing experiments were performed to evaluate the combined effect of 1 wt% fumed silica + 0.1 wt% H2O2 + 0.02 M oxalic acid solution together with 5 ppm imidazole on removal rate selectivity of Co and Cu.
A similar case where the use of inhibitors suppresses or flattens the peak is observed in the literature (Idouhli et al., 2019). A similar almost negligible shift in the UV spectrum is observed with imidazole treatment with different components. (Bui et al., 2012) The experiments were performed with bulk Co and Cu metal disk with 99.98% and 99.95%.
Anodic dissolution of Cobalt in hydrogen peroxide solutions with and without complexing agent: Kinetic analysis by electrochemical impedance spectroscopy
- Motivation
- Experimental Conditions Electrochemical experiments
- Results and Discussion Anodic polarization studies
The decrease in OCP value clearly shows that the oxide/hydroxide layer formed on the Co surface in H2O2 solution dissolves with the addition of oxalic acid. It is therefore clearly evident that the Co anodic dissolution rate is higher in H2O2 +oxalic acid solution than in H2O2 solution. The surface of Co treated in H2O2+oxalic acid solution, as observed, mainly consists of small but uniformly distributed tubules/granules/flakes.
The decrease in contact angle with addition of oxalic acid indicates the formation of complexes with Co oxides/hydroxides. This indicates the possibility of the presence of combination of Co-oxalate complexes together with the CoOOH /Co3O4 or the presence of individual complexes or oxides when treated in H2O2-oxalic acid solution. A precise spin-orbit peak at 532.6 eV attributes to the presence of Co-oxalate complexes.
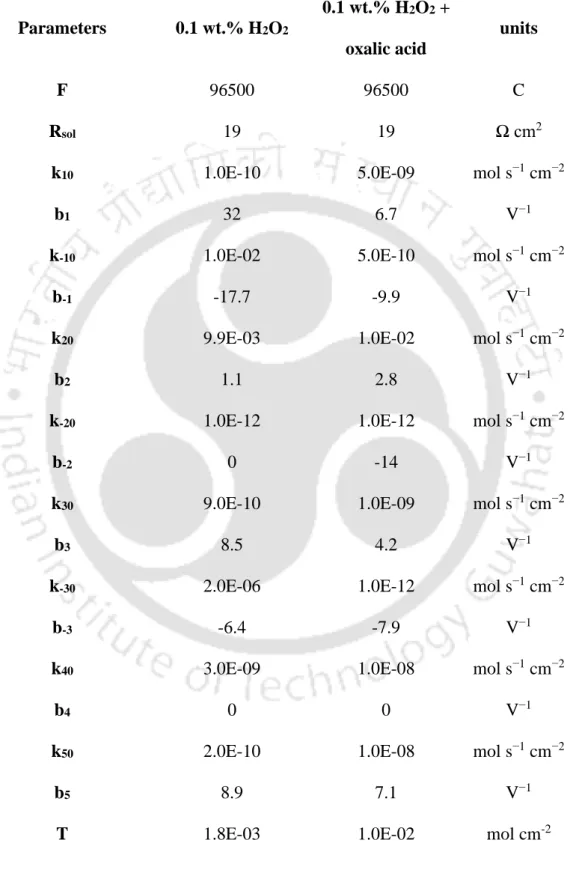
A decrease in peak intensities is observed for all the compounds formed from Co treated in H2O2-oxalic acid system. The equivalent circuit proposed for the systems is illustrated in Figure 4.4.6(a). The EEG simulated and experimental EIS curve are displayed in Figure 4.4.6(b) and Figure 4.4.6(c), while the best fitted EEG parameters with percentage error of less than 5%.
- wt.% H 2 O 2 + oxalic
- wt.% H 2 O 2 + oxalic acid
Higher Rp values of the H2O2 system indicate more adsorbed species present on the Co surface. While EEG model fitting provides an insight into the decomposition process; detailed anodic decomposition together with total surface coverage of the adsorbed species cannot be explained by it. The reaction mechanism analysis (RMA) method was performed to get a clear picture of the reaction pathway (Keddam, Mottos, and Takenouti, 1981; Maddala et al., 2010; Lee et al., 2016).
Recently, Paul et.al (Paul and Srinivasan, 2020) studied the dissolution of Co in an alkaline glycine solution. Thus, the following mechanism {Refer to Equation (4.4.23 and 4.4.24)} is suggested to explain the impedance patterns observed for both H2O2 and H2O2 + oxalic acid solutions. Based on the Pourbaix diagram, (Pourbaix, 1974) 𝐶𝑜3+ is considered as the dissociation species instead of 𝐶𝑜+and 𝐶𝑜2+ at pH 9 and the applied potential.
As reported in the literature, all adsorption models lead to a fixed time constant and in the proposed mechanism, the Langmuir adsorption isotherm model was adopted. (Keddam, Mottos and Takenouti, 1981). To determine the faradic impedance, the current density is differentiated by the voltage which is shown in the following equation.