Characteristic curling of the root hairs and invasion of the bacteria to form a thread of infection. Release of the bacteria from the infection thread and their differentiation as specialized nitrogen-fixing cells. The infection thread continues to elongate until it reaches the base of the root hair cell.
AMMONIA ASSIMILATION
Catalytic
Two enzymes glutamine synthetase and glutamate synthase work in tandem to form the glutamate synthase cycle of ammonia assimilation. When NH4+ is limiting, most of the glutamate is made by the sequential action of glutamine synthetase and glutamate synthase.
LIPID STRUCTURE AND FUNCTION
ALCOHOLS
FATTY ACIDS
- H COOH
- CH 2 ) 5 CHCH 2 CH=CH(CH 2 ) 7 COOHOH
- CH 2 ) 21 CH–COOH Cerebronic acid
- CH 2 ) 7 CH––CH(CH 2 ) 7 COOH
- CH 2 ) 7 CH CH(CH 2 ) 7 COOHO
- CH 2 ) 5 CH CH(CH 2 ) 9 COOHCH2
- CH 2 ) 7 C C(CH 2 ) 7 COOHCH2
Monoethenoic acids are commonly referred to as monounsaturated fatty acids (MUFA) and the remaining polyunsaturated fatty acids are classified under polyunsaturated fatty acids (PUFA). In most unsaturated fatty acids, there is a double bond (designated ∆9) between carbon atoms 9 and 10. Due to the presence of double bonds, unsaturated fatty acids exhibit geometric (or cis-trans) isomerism. .
A double bond in a zigzag line is indicated by drawing an additional line between the carbon atoms involved in the formation of the double bond. When a cis double bond is inserted (as in oleic acid), the molecule bends and takes the shape shown below. Another structural feature of naturally occurring polyunsaturated fatty acids is the presence of an unconjugated double bond system.
This fatty acid may be due to the addition of a methylene group over the double bond of vaccenic acid (18:1; 11). It can be derived from oleic acid by the addition of a methylene group across the double bond in a way that leaves the unsaturated nature unchanged, unlike lactobacillilic acid.
FUNCTIONS OF LIPIDS
Similarly, sterculic acid from plant sources has a comparable structure, with a suggested relationship to oleic acid.
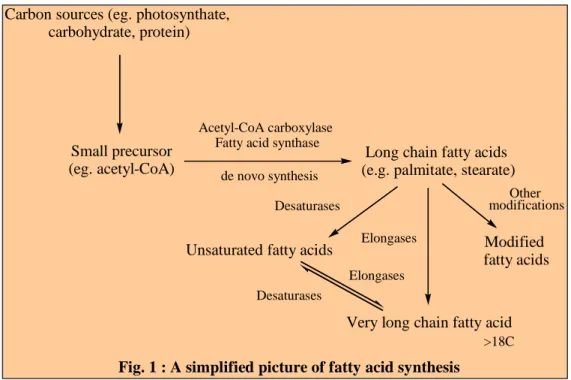
LIPID BIOSYNTHESIS
Biosynthesis of Fatty Acids
Acetyl CoA transport into the cytosol
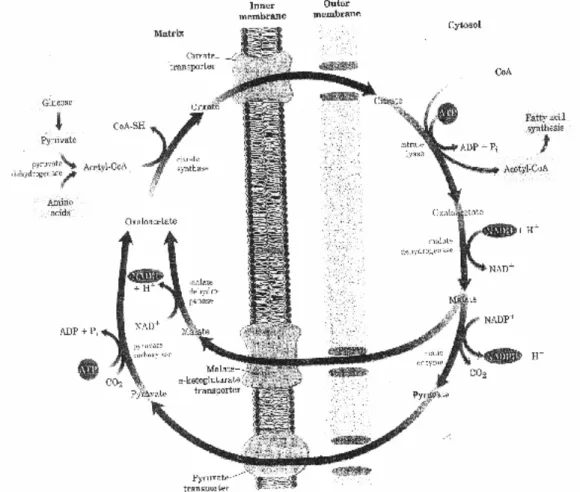
Figure 3 Acetyl-CoA as a key intermediate between fat and carbohydrate metabolism. Arrows indicate the main pathways of acetyl-CoA generation or use. Citrate serves as a carrier for the transport of acetyl units from the mitochondrion to the cytosol for fatty acid synthesis.
Production of Malonyl CoA : The initiation Phase
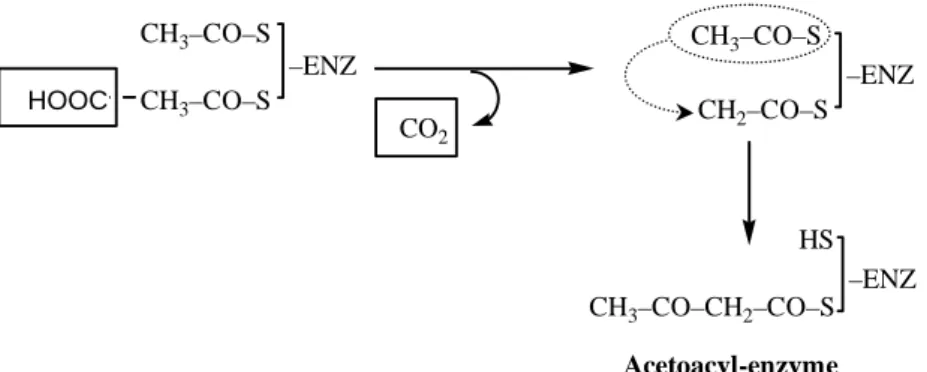
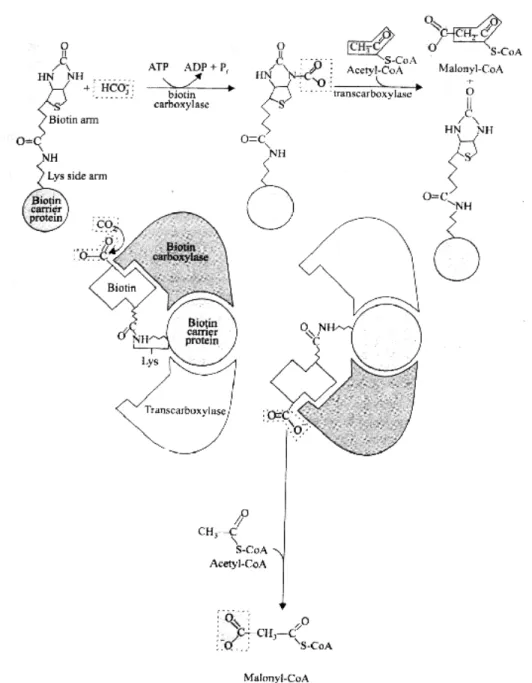
First Step -: The biotin carboxylase (BC) catalyzes carboxylation of biotin carboxyl carrier protein (BCP) to yield carboxybiotin carboxyl carrier proteins (BCP-COO–); the carboxyl group is derived from bicarbonate (HCO3–). Upper figure, the fatty acid binds to the prosthetic group by forming a thioester bond with the sulfhydryl group. In other words, the –SH group is the site of entry of malonyl groups during fatty acid synthesis.
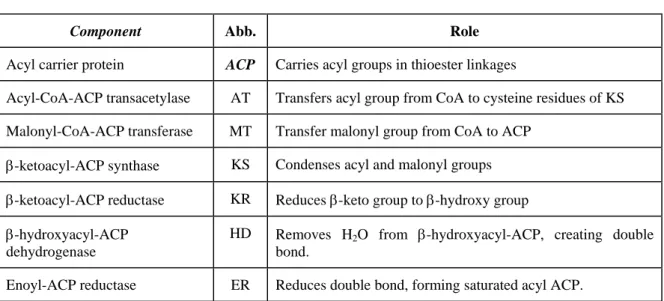
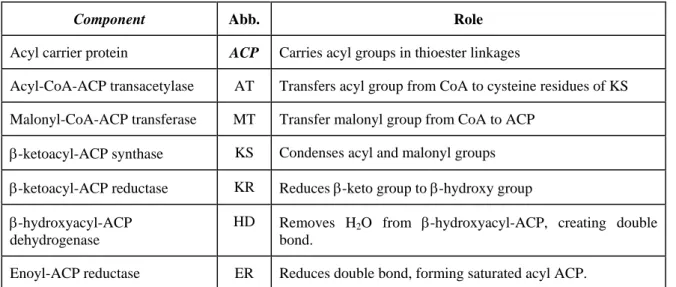
The 4'-phosphopantetheine prosthetic group in ACP serves as a flexible arm that binds the growing fatty acyl chain to the surface of the fatty acid synthase complex and carries the reaction intermediate from one enzyme active site to the other. All the reactions in the biosynthesis of fatty acids are catalyzed by a multienzyme complex, the fatty acid synthase. The other -SH group carries the extended chain as it undergoes the reaction necessary for reduction to a saturated acyl group, and it also accepts the acetyl and malonyl groups from which the fatty acid is built.
The 'central' one is the –SH group of acyl carrier protein (ACP), with the intermediates of fatty acid synthesis forming a thioester and the 'peripheral' one is the –SH group of a cysteine residue in β-ketoacyl-ACP synthase, one of the 7 proteins of the multi-enzyme complex. The bacteria therefore contain separate proteins to catalyze the individual reactions of fatty acid synthesis, even the formation of mlonyl-CoA takes place in 2 stages (carboxylation of biotin and transfer of the -COO– group to acetyl-CoA).
The Fatty Acid synthase in some Organisms
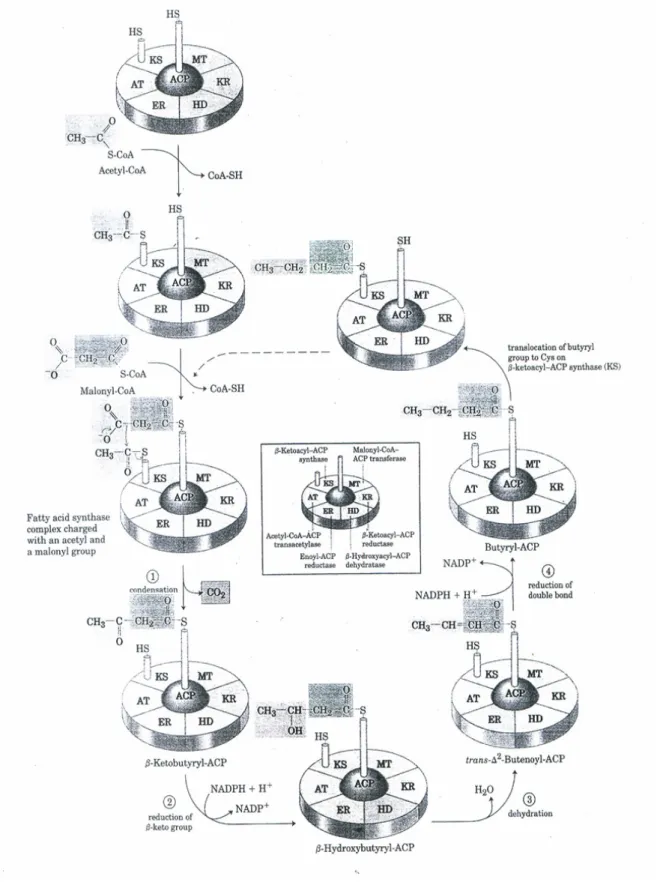
Acyl-CoA-ACP transacetylase AT Transfers the acyl group from CoA to cysteine residues of KS Malonyl-CoA-ACP transferase MT Transfers the malonyl group from CoA to ACP. The fatty acid synthases of yeast and vertebrates are also multienzyme complexes, but their integration is even more complex than that in E. Relative molecular mass, Mr contains all seven enzymatic activities, as well as a hydrolytic activity that separates the fatty acid from the ACP-like component of the enzyme complex.
Each segment of the disc represents one of the 6 enzymatic activities of the complex: acetyl-CoA-ACP transacetylase (AT); malonyl-CoA-ACP transferase (MT); β-ketoacyl-ACP synthase (KS), which contains a critical Cys-SH residue; β-ketoacyl-ACP reductase (KR); β-hydroxyacyl-ACP dehydrase (HD); and enoyl-ACP reductase (ER). In the middle is an acyl carrier protein (ACP) with its phosphopantheine arm (Pn) ending in another –SH. Before the condensation reactions (the built-up fatty acid chain) can begin, the two –SH groups on the enzyme complex must be loaded with the correct acyl groups.
In the first step, the acetyl group of acetyl-CoA is transferred to the cysteine SH group of the β-ketoacyl ACP synthase. In the second step, the malonyl group of malonyl-CoA is transferred to the –SH group of ACP by the enzyme malonyl-CoA-ACP-transferase, also part of the complex.
The Phase of Elongation
Reduction of the Carboxyl group
The acetoacetyl group formed in the condensation step then undergoes reduction of the carboxyl group at C-3 to form D-β-hydroxybutyl-ACP. This reaction differs from the equivalent in fatty acid degradation in two respects. a) The D rather than L eprimer is formed. This difference exemplifies the general principle that NADPH is consumed in biosynthetic reaction, whereas NADH is generated in energizing reaction.
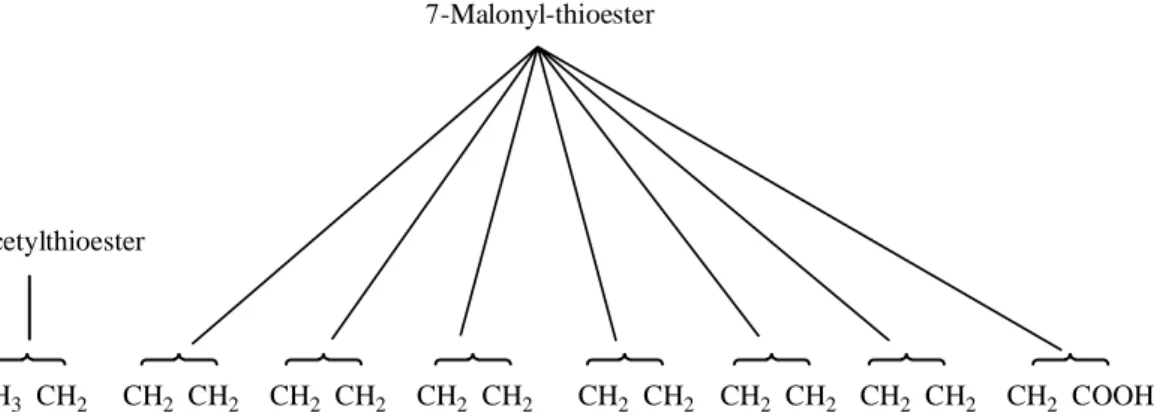
In the third step, water elements are removed from C-2 and C-3 of d-β-hydroxybutyryl-ACP to form a double bond in the product, trans-∆2-butenoyl-ACP (also called crotonyl-ACP). . Finally, the double bond of trans-∆2-butenoyl-ACP is reduced, reduced (or saturated) to form butryl-ACP by the enzymatic action of enoyl-ACP reductase; again, NADPH is the electron donor on the reductant. Thus, after the first round of C4 (butryl) elongation, the palmitate precursor was synthesized from C2 (acetyl) and C3 (malonyl) units, with the acetyl group forming the two terminal carbons of the growing fatty acid chain (C15 and C16 in palmitate, for example).
The general sequence of condensation and reduction by fatty acid synthase can be represented schematically as.
Successive Rounds
Stoichiometry of Fatty Acid Synthesis
Acetate is shuttled out of Mitochondria in the form of Citrate
Acetyl groups exit the mitochondrion as citrate; in the cytosol they are delivered as acetyl-CoA for fatty acid synthesis. Intra-mitochondrial acetyl-CoA first reacts with oxaloacetate to form citrate, in the citric acid cycle reaction catalyzed by citrate synthase. In the cytosol, citrate is cleaved by citrate lyase which regenerates acetyl-CoA in an ATP-dependent reaction.
Alternatively, malate produced in the cytosol is used to generate cytosolic NADPH through malic enzyme activity. A picture. Mammals cannot convert oleate to linoleate or linolenate, so they are required in the diet as essential fatty acids (EFAs). Electron transfer pathway in fatty acid desaturation by a mixed-function oxidase in vertebrates.
The path of electron flow involves a cytochrome (cytochrome b5) and a flavoprotein (cytochrome b5 reductase), both of which, like fatty acyl–CoA desaturase itself, are present in the smooth ER.]. Mammalian hepatocytes can readily introduce a double bond at the Δ9 position of fatty acids, but cannot introduce an additional double bond into the fatty acid chain between C-10 and the methyl terminal end.
Families of Fatty Acids
There are 4 series of unsaturated fatty acids in mammals: two are derived from dietary linoleate and linolenate, and the other two are synthesized from the monounsaturated fatty acids, oleate and palmitoleate, which are formed from the corresponding saturated fatty acids. The four series can be recognized by the distance between the terminal methyl group (ω) and the nearest double bond. Note that the precursor family and its products made in animals through elongation and desaturation can be identified by subtracting the number defining the smallest double bond from the total number of carbon atoms.
DEGRADATION OF FATTY ACIDS
Electron transfer
In this step, a mole of water is added to the double bond of trans-∆2-enoyl-CoA to form the L-stereoisomer β-hydroxyacyl-CoA (also called 3-hydroxyacyl-CoA). The reaction is catalyzed by enoyl-CoA hydratase or crotonase and has broad specificity for the length of the acyl group. However, its activity gradually decreases with increasing substrate chain length (it can be seen that the enzyme will also hydrogenate α, β-cis unsaturated acyl-CoA, but in this case we find D (–)-β-hydroxyacyl-CoA).
In this step, L-β hydroxyacyl-CoA is dehydrogenated (or oxidized) to form β-ketoacyl-CoA by the action of an enzyme, β-hydroxyacyl-CoA dehydrogenase, which is completely specific for the L-stereoisomer hydroxyacyl substrate. NAD+ is the electron acceptor in this reaction and the NADH thus formed donates its electrons to NADH dehydrogenase, the electron carrier in the respiratory chain. The reaction catalyzed by β-hydroxyacyl-CoA dehydrogenase is very similar to that of citric acid malate dehydrogenase.
The net result of the first three reactions is the oxidation of the methylene group at the β(or C-3) position to retain the substrate group, acyl-CoA. The shortened acyl-CoA then undergoes another cycle of oxidation, starting with the reaction catalyzed by acyl-CoA dehydrogenase β-ketothiolase, hydroxyacyl dehydrogenase, and enoyl-CoA hydratase all have broad specificity with respect to the length of the acyl group.
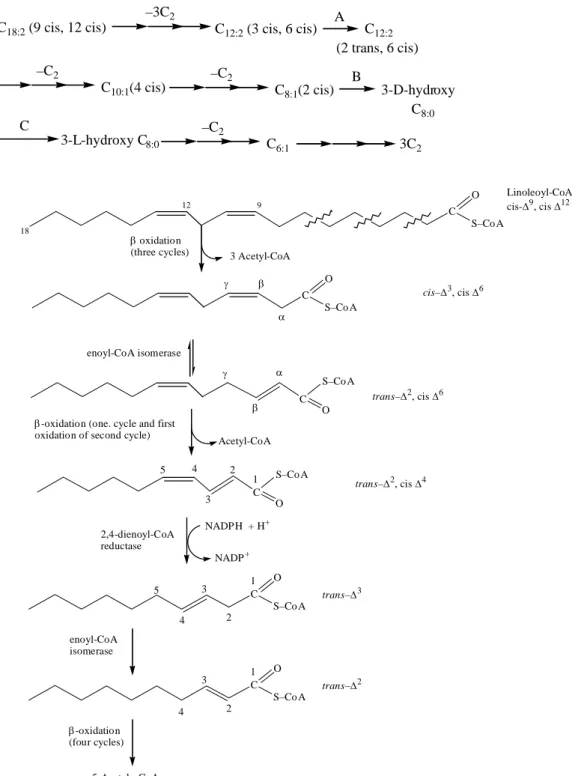
OXIDATION OF UNSATURATED FATTY ACIDS
However, the combined action of enoyl-CoA isomerase and 2,4-dienoyl-CoA reductase fig (5) allows the re-entry of this intermediate in the typical β-oxidation pathway and its degradation to 6 acetyl-CoAs. The introduction of 2 additional types of enzymes (an enoyl-CoA isomerase and a 3-hydroxyacyl-CoA racemase) allows any combination of double bonds found in an unsaturated chain to be handled through the same pathway used for saturated fatty acids. The roles of the 3 additional enzymes required for the oxidation of a dienoic acid (or polyenoic acid) can be summarized here, where A is enoyl-CoA isomerase, B, enoyl-CoA hydratase and C, 3-hydroxyacyl- CoA epimerase, monoenoic acid, and dienoic acid are oxidized at comparable rates.
5 Oxidation of unsaturated fatty acids requiring two additional enzymes, enoyl-CoA isomerase and 2,4-dienoyl-CoA reductase (Note that the combined action of these two enzymes converts a trans ∆2 intermediate, cis ∆4-dienoyl- CoA to the substrate trans-∆2- enoyl-CoA, required for β-oxidation.).
OXIDATION OF ODD-CHAIN FATTY ACIDS
CH COO –
Carnitine