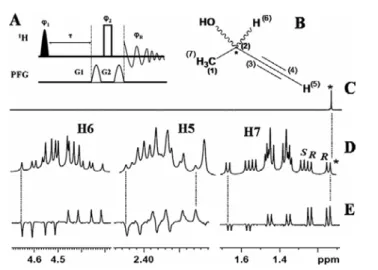
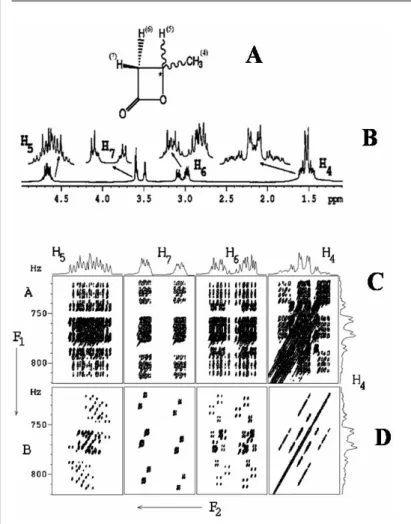
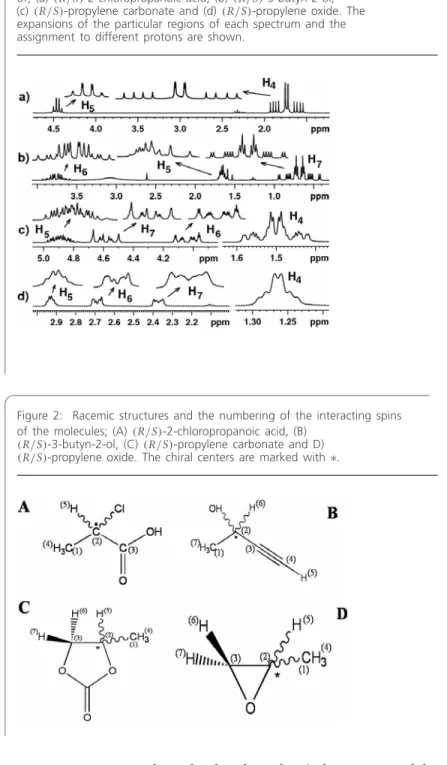
There is an anisotropic interaction between the polypeptide helices of PBLG and the enantiomers. One of the A3 sub-spectra corresponds to the methine proton in the |α state and the other to the methine proton in the |β state. An isolated peak (marked in the figure) or a pair of peaks is observed in the high-field region of the methyl group of the spectrum.
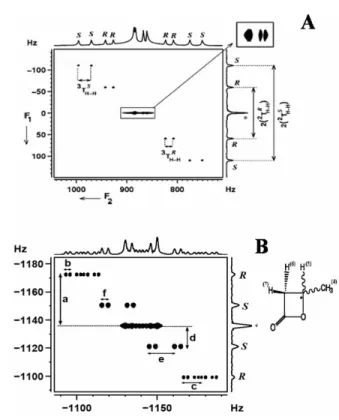
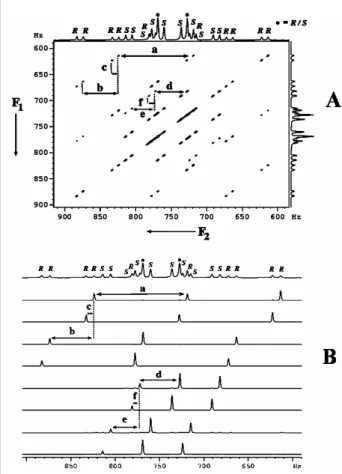
The selectivity of the excitation of a single transition from the one-dimensional spectrum is reported in Fig. The processes that take place after the application of the mixing pulse can be written as. Because the separation TAAint1 dimension is different for the Randzene enantiomers, there is chiral discrimination visualized for the outer components of the triplet.
Therefore, there is a diagonal skew of the peaks (analogous to the derived 2D J-resolved spectrum) in such a 2D correlation matrix. DQ-selective excitation of methyl protons and application of a selective 180◦ refocusing pulse in the middle of the 1-dimension results in an AX spin system in the DQ dimension.
Single quantum two dimensional methods
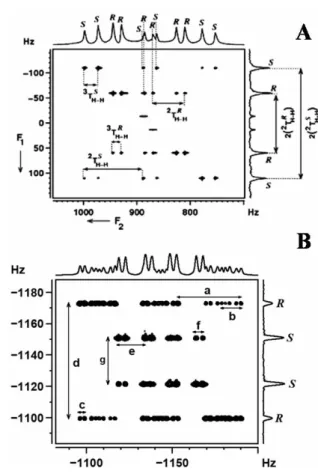
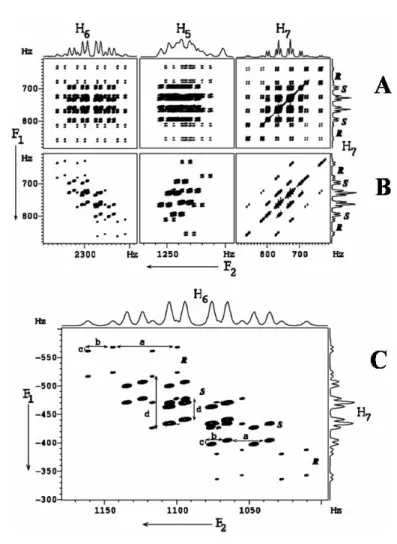
The DQ dimension provides 3 × (2DH H) for both enantiomers, thereby increasing the resolution for better discrimination;. B) 500 MHz 2D DQSERF spectrum of (R/S)-β-butyrolactone in PBLG, relating the DQ coherence of the methyl protons to its SQ coherence together with the corresponding projections. Coupling information can be obtained from cross-peak multiplet pattern analyses. From the expanded cross-peak plot for the methine proton (6) (Figure 9B), it can be seen that the passive coupling to proton 6,.
The analysis of the spectrum yields one active linkage, 3TH5H7, and four passive links, 2TH6H7, 3TH5H6, 3TH4H5and. An extended region of the spectrum related to the proton 7 cross peaks is shown in Fig. Similar information is available from the cross-peak region of the spectrum corresponding to proton 5.
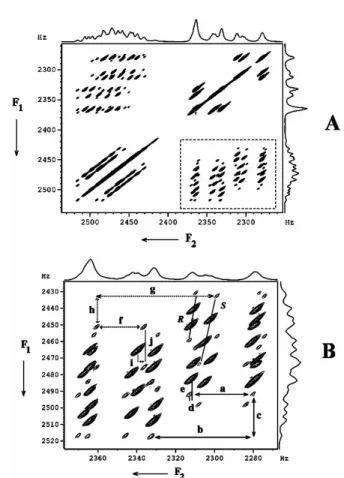
The BASE-COSY experiment is a variant of the COSY experiment in which a small band of frequencies is selectively excited in the t1 dimension and correlated with the entire spectrum in the t2. The significant advantage of BASE-COZY experiment is therefore the zooming in of the small area of the spectra in the F1 dimension which offers high. The disadvantage of BASE-COSY is that the complexity persists in the F2 dimension and the analyzes of the spectra become very tedious with too many transitions in each section.
The displacement of the passive couplings in dimensions F1 and F2 also enables the measurement of couplings smaller than the line width. One of the ways to circumvent this limitation is to call that part of the spectrum in the indirect dimension56,57. Note: in H7, a doublet of small coupling appears as a broad hump in the BASE-β-COSY spectrum, clearly bringing out the advantage of the BASE-z-COSY experiment.
This is evident from the spectrum broadenings for the H5 proton for both BASE-β-COZY and BASE-z-COZY given in Figs. Its z-COSY spectrum and the expansions of different regions of the spectrum for the assigned protons are given in Figs. In the reported study, T1 values of protons were measured using the standard inversion recovery method.
Triple quantum two dimensional methodology
Numbers 1 and 2 are pairs of contours of the individual multiple component belonging to each enantiomer. The magnitudes of these displacements provide the passive couplings and (iv) the animations of the displacement vectors provide the relative signs of the couplings. The expansion of the two contours of the 2D matrix shown clearly identifies the two peaks of the enantiomers.
Due to the magnetic equivalence of the methyl protons, this value is also increased threefold in the 3Q dimension, which can be seen after discrimination by comparing the center of the two separate Rand spectra. In (R/S)-3-butyn-2-ol, when all three methyl protons are rotated, the spin system in the 3Q dimension is of the AMX type, where A is a super spin with three methyl protons, M is a methine proton, and X is respectively acetylenic proton. Corresponding to the four spin states|αα,|αβ,|βαand|ββ of the passive spins M and X, there are four allowed 3Q transitions for spin A arising from each enantiomer, as opposed to 12 transitions in a SQ spectrum.
23, is concerned, the spin systems are of type AMPX in the 3Q dimension, where A is the super spin with 3 methyl protons and the passive spins M, P and X are protons. Active connections are present in every cross section, while passive connections are responsible for shifting the subspectra. Using two selectively detected 3Q-SQ experiments, complete analyzes of the superimposed spectra of both enantiomers in six spin systems, ie, (R/S)-propylene carbonate and (R/S)-oxide, have been performed propylene.
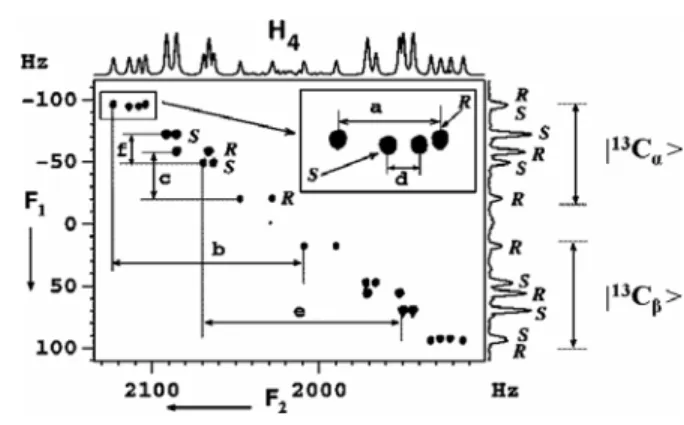
In methyl group excitation experiments, the resolution in the 3Q dimension is 3(nTH H) and one in the SQ dimension results in a total 3.3-fold chiral dispersion in the tilted direction as far as the measurement of resonance frequencies between chiral molecules is concerned. Due to the large width of the contours along the F1 axis, the less intense peaks of the Renantiomer are also visible in the cross-section. The difference in σi between the two enantiomers also increases threefold in the 3Q dimension, as can be seen from the difference in the mean doublet frequencies of the two enantiomers in (R/S)-2-chloropropanoic acid (Figure 22A).
The comparison of the tilt directions of the multiplets in different cross-sections for different chemical shift positions gives the relative signs of the couplings.
Chiral discrimination in the absence of the dipolar or quadrupolar field
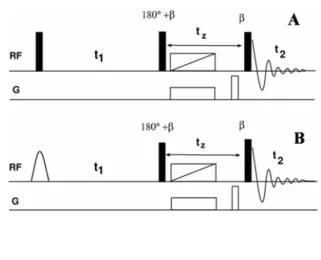
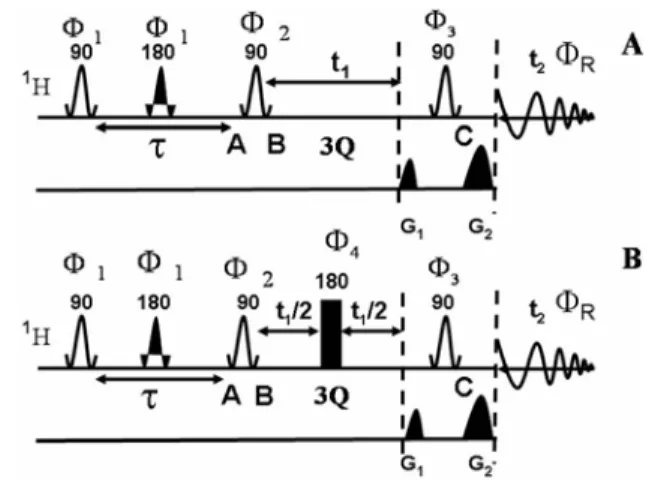
One dimensional spectrum given below the 2D spectrum is the cross section taken along SQ dimension at the position of S enantiomer in the 4Q dimension. In favorable cases, the parameters derived from the 2D spectra can be used for the iterative analysis to derive all other spectral parameters. The behavior of the magnetization during different stages of pulse sequences is discussed in the text.
The delay τ1 responsible for polarization transfer depends on the factor 1/(4(1TAX)) and was adjusted for each molecule independently, the gradient ratio used was G2:G3= 1:2.
The broadening of a small region of the spectrum given in the inset depicts the resolution of closely resonant transitions. In the middle of the 1st dimension, the simultaneous application of a selective refocusingπpulse on the methyl protons and a non-selectiveπpulse on 13C conserves 1TCHand. The spectrum in the direct dimension corresponds to the spin system of type A3MX, which is a doublet of the doublet of a triplet.
The methyl protons in the direct dimension experience three different couplings, namely to each other (2TH H), to the methine proton (3TH H) and to 13C of the methyl group (1TCH). In the CH-SERF experiment, as a consequence of the introduction of 1TCH is an additional parameter with their significantly different values, there is complete distinction between enantiomers. The last selective 90◦y pulse on methyl protons results in the observable SQ magnetization in the direct dimension at time F.
The 3Q exorcycle phase cycling was employed for non-selective 1800 pulses in the center of 1 dimension. During the generation of 3Q coherence and its evolution and conversion to SQ coherence, the spin states of the remaining protons (passive spins) in the molecule and 13C spin of the methyl group are unperturbed in both dimensions. The selective excitation of 3Q coherence of the methyl protons leads to the simultaneous flipping of all the protons.
Thus, the spin system in the 3Q dimension will be of the AMPX type, where A is a super spin with three methyl protons. In the 3Q dimension, the maximum coupling to spin A occurs due to JCH+2DCH, where JCH and DCH are scalar and dipolar coupling between the proton and the 13C methyl group. Only cross-sections relating to the β(13C) regions are given. The R and S assignments and the passive spin states of the protons in the 3Q dimension are shown.
Methyl protons undergo five different types of coupling viz. a) mutual couplings (2TH H), (b) couplings between methyl and methine protons (P) (3TH H), (c) two different couplings of two diastereomeric methylene (M and N) protons (4TH H ) and (d) coupling from spin 13C (X).
Conclusions
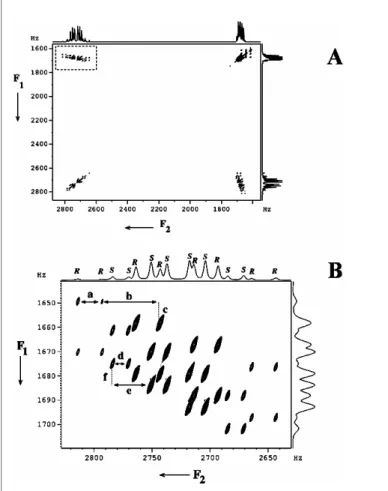
Note the advantage of spin mode selection in distinguishing even the peaks marked with * in Fig. 33a. The selective methyl protons excited, 13 C edited 2D 3Q-SQ correlation spectrum of (R/S)-propylene carbonate is given in Fig. Superspin A is the three methyl protons (the active spin), the methine and methylene protons (M), N and P) and one13C spin (X) make up the passive spins.
The 3Q dimension provides sixteen transitions for each enantiomer, corresponding to the sixteen spin states M, N, P, and X. Analyzes of the spectra at any 13C spin state are sufficient to derive all homonuclear couplings with methyl protons. For the Senantiomer, the two 4TH Hare types are identical and therefore result in triplet doublets (due to 3TH H) in the 3Q dimension and are labeled S.
So every cross section along the SQ dimension for every passive spin state in the 3Q dimension is a triplet. It can be pointed out that the coupling information between the numbered protons 5, 6 and 7 is reflected neither in the 3Q nor in the SQ dimension. In addition to all the advantages of the previously described method, DCH can also be derived.
Spectral widths of 535 Hz and 300 Hz were selected in the direct and indirect dimensions. The spectrum was displayed in magnitude mode with a digital resolution of 0.15 Hz and 0.52 Hz in the direct and indirect dimensions, respectively. The displacements of the α(13C) and β(13C) regions in the F2 dimension that provide heteronuclear passive couplings are indicated as a and f for the Sand and R enantiomers, respectively.
The feasibility of methods for the accurate determination of enantiomeric excess was also discussed.
Acknowledgements
Separations labeled b–e and g–j provide proton–proton coupling information, and the corresponding values are listed in Table 1.