Their valuable inputs and evaluations during my research progress kept the flow of research work in the right direction. In addition, I would like to thank other professors and staff members of the Department of Chemical Engineering for their valuable support during my research.
ABE Fermentation
Chapter 1: Introduction 3 Biomass with higher sugar and starch content provides a better feedstock for butanol production [2]. Various separation techniques such as distillation, extraction, degassing, adsorption, and evaporation were previously used for the extraction and upgrading of butanol from the ABE product stream [ 2 , 7 , 8 ].
![Figure 1.1: Energy Requirements for Butanol Removal by Various Separation Techniques [9]](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/29.892.169.774.382.913/figure-energy-requirements-butanol-removal-various-separation-techniques.webp)
Ionic Liquids
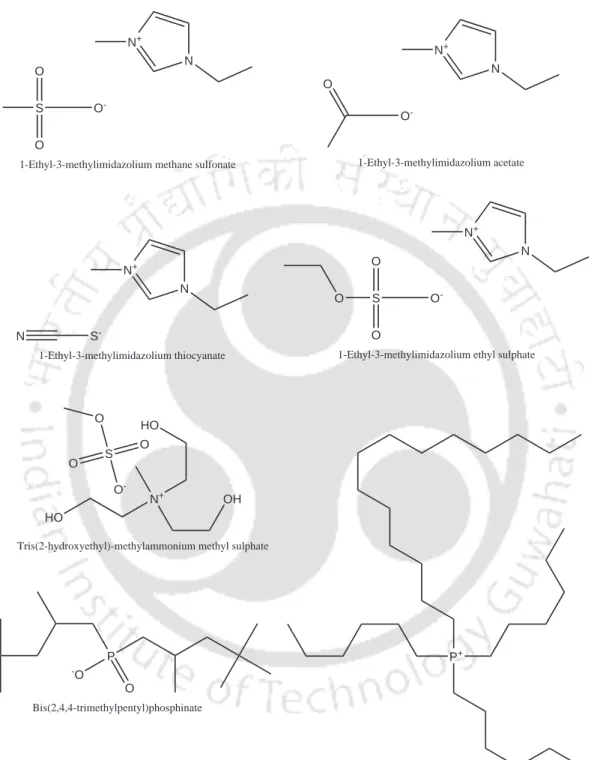
Different types of room temperature ILs are based on cations such as alkylammonium, tetraalkylammonium, tetraalkylphosphonium, 1,3-dialkylimidazolium and N-alkylpyridinium. Cations such as 1-alkylpiperidinium and 1,3-dialkylimidazolium salts are formed with anions that have low nucleophilicity, such as bis(trifluoromethylsulfonyl)imide, hexafluorophosphate, tetrafluoroborate, perfluoroalkylsulfonate, thiocyanate-methylsulfonate, thiocinate and thiocinate-methylsulfonate, thiocinate.
Butanol Enhancement using Ionic Liquids
22] have shown that fewer steps are required in the extraction of 1-butanol from aqueous solution using 1-hexyl-3-methylimidazolium tris(pentafluoroethyl) trifluorophosphate ([HMIM][eFAP]) compared to 1-(6-hydroxyhexyl )-3-methylimidazolium bis-(trifluoromethylsulfonyl)imide ([HOhMIM][Tf2N]). Chapter 1: Introduction 9 Kubiczek and Kamiński [51] extracted all three components, namely acetone, butanol and ethanol from water using 1-hexyl-3-methylimidazolium hexafluorophosphate ([HMIM][PF6]) and 1-methylimidazolium-3. (trifluoromethylsulfonyl)imide ([BMIM] [Tf2N]).
Objectives
Some phosphonium-based ILs are lighter than water and they have been used for the extraction of organic components from aqueous solution [53–55] . We have optimized the number of steps and the solvent flow rate by minimizing the multistage extractor cost for the extraction of butanol and ethanol from aqueous solution using phosphonium-based ILs.
Organization
Chapter 1: Introduction 13 Chapter 4 presents the experimental liquid-liquid equilibrium data for the quaternary systems, namely [TDTHP] [Phosph]-ethanol-butanol-water and [TDTHP]. Chapter 5 describes the optimization of multi-step extractor from the binary interaction parameters which were generated by NRTL model using experimental LLE data as obtained in Chapter 4.
Introduction
Chapter 2: Properties of the pure ionic liquid 19 decanoate [TDTHP][DEC] at T=298.15 K by classical molecular dynamics simulation (Section 2.3.2).
Experimental Methodology
Computational Details
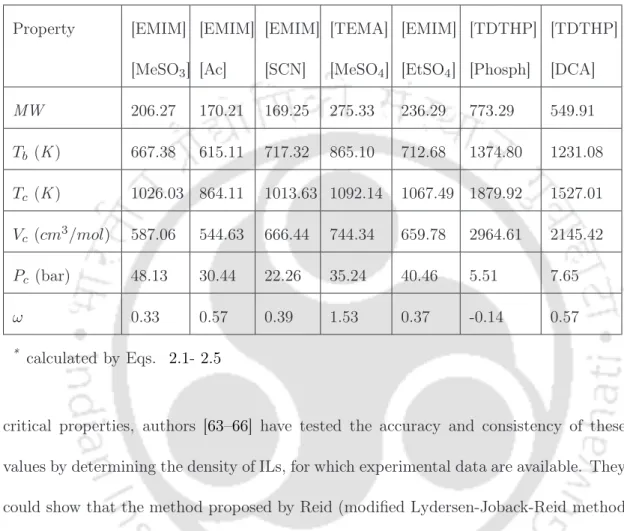
Chapter 2: Pure ionic liquid properties 23 According to the literature [58], these seven models show minimal deviation from the experimental values of density and proved to be a better alternative for density estimation. Chapter 2: Pure Ionic Liquid Properties 28 where the individual terms correspond to the bonding interactions such as bonds, angles, dihedrals (the first three terms) and the non-bonded interactions such as van der Waal and electrostatics (the fourth and fifth terms).
Results and Discussion
The NAMD configuration file, PDB file, PSF file, and force field parameter file for both ILs are shown in APPENDIX A. Chapter 2: Pure Ionic Liquid Properties 29. Chapter 2: Pure Ionic Liquid Value 31M2 with the values of 31M processable for the values of 31M2PRimit2. M1 and NMSRK models.
![Figure 2.3: Comparison of experimental density of [EMIM][SCN] with literature values [73]](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/55.892.162.799.140.930/figure-comparison-experimental-density-emim-scn-literature-values.webp)
Introduction
Hydrogen in the aromatic functional group gives a chemical shift peak at more than 7 ppm while aliphatic hydrogen gives a peak less than 7 ppm. In the case of butanol separation from phosphonium ILs, there is a lack of aromatic hydrogen in all systems. So, we have performed a comparison system, namely, the aliphatic-aromatic separation by ammonium and phosphonium ILs.
In addition to aromatic-aliphatic separation, we considered the separation of lower alcohols such as 1-propanol from an aqueous solution using [TDTHP][Phosph] as a solvent. Having gained sufficient confidence in the characterization by NMR spectroscopy, we focused on the extraction of butanol by three different ILs, namely [TDTHP][Phosph], [TDTHP][DCA].
Chemicals and Materials
ILs namely Tris (2-hydroxyethyl) methylammonium methylsulfate [TEMA][MeSO4] and Tributyl methyl phosphonium methyl sulfate [P4441][MeSO4] (Fig.3.1) were chosen for this purpose. To remove impurities, the ILs were immersed in an oil bath for 24 h at 353 K. 52 Chapter 3 Ionic Liquids as an Extraction Media in Ternary Systems Table 3.1: Purity of chemicals and purification methods.

Aromatic-Aliphatic Separation by ILs
Chapter 3: Ionic liquids as an extraction medium in ternary systems 54 with the sample (0.1 ml) in NMR tubes (degree of economy). Chapter 3: Ionic Liquids as an Extraction Medium in Ternary Systems 57 Here xi is the mole fraction of samples and Hi is the single hydrogen peak area for component. Chapter 3: Ionic liquids as an extraction medium in ternary systems 59 Table 3.2: Experimental line data for [TEMA][MeSO4] (1) -Benzene (2) - Hexane.
The [P4441][MeSO4]-based ternary system studied in this work (Table 3.4) contains a negligible IL concentration of 0.006 mole fraction in the raffinate phase. Chapter 3: Ionic liquids as extraction media in ternary systems 60 Table 3.3: Experimental coupling data for [TEMA][MeSO4] (1) -Toluene (2) - Heptane. However, for the [TEMA][MeSO4](1)-toluene (2)-heptane (3) system (Table 3.3), the partition coefficients showed no specific trend.
![Figure 3.2: Raffinate phase 1 H NMR spectra for the system: [TEMA][MeSO 4 ] (1) - -Benzene (2) - Hexane (3) at T =298.15 K and p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/81.892.178.767.196.1043/figure-raffinate-phase-spectra-tema-meso-benzene-hexane.webp)
Butanol Extraction by Phosphonium based ILs
68 Chapter 3 Ionic liquids as extraction media in ternary systems Table 3.5: Experimental coupling line data for the system [TDTHP][Phosph] (1). This is due to the higher polarity of propanol compared to butanol, which results in better water solubility. The distribution coefficient varied in the range of 25-95 and 17-173 for ternary systems namely [TDTHP][DCA].
The distribution coefficient for [TDTHP][DCA] is twice as high as the ILs reported by Nann et al. The distribution coefficient for the current three systems is compared with literature data [49] in Fig.3.19. It implies that [TDTHP][DCA] and [TDTHP][DEC] are completely immiscible with water in a ternary system.
![Figure 3.9: Extract phase 1 H NMR spectra for the system: [TDTHP][Phosph] (1) - -1-Butanol (2) - Water (3) at T =298.15 K and p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/90.892.120.729.171.1055/figure-extract-phase-spectra-tdthp-phosph-butanol-water.webp)
LLE Modeling
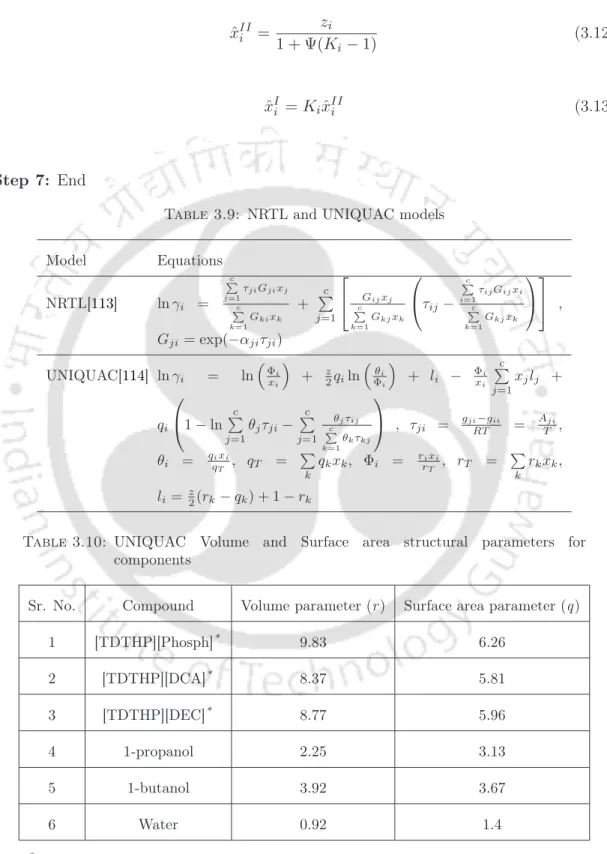
Chapter 3: Ionic Liquids as an Extraction Media in Ternary Systems 77 Step 6: Calculate the mole fractions in both phases. Chapter 3: Ionic liquids as an extraction media in ternary systems 78 These predicted concentrations are included with GA [116] to minimize the objective function (Eq. 3.14). Chapter 3: Ionic Liquids as an Extraction Media in Ternary Systems 79 deviation from the experimental data of the tie line as shown in Eq.
Chapter 3: Ionic liquids as an extraction medium in ternary systems 84 3.5.2 COSMO-SAC model (conductor-like screening model-. Segment activity coefficient). Chapter 3: Ionic liquids as an extraction medium in ternary systems 87 The exchange energy contains contributions from electrostatic interactions, referred to as mismatch energy Emf, and is calculated by electrostatic interactions between a pair of segments and hydrogen bonding energy Ehb, which occurs when an electropositive atom (hydrogen) forms additional bonds with an electronegative atom such as oxygen, nitrogen or fluorine. Chapter 3: Ionic liquids as extraction media in ternary systems 88 This equation is solved iteratively.
Introduction
Experimental Procedure
Chapter 4: Simultaneous extraction of ethanol and butanol from aqueous solution 92 same molar ratio between ethanol and butanol (0.268), but different molar ratio between water and IL (9th, 10th and 11th tie line in Table 4.1). From both figures, it can be seen that few tie lines point towards the ethanol peak, which represents more extraction of ethanol compared to butanol. Chapter 4: Simultaneous extraction of ethanol and butanol from aqueous solution96 Table 4.1: Experimental tie lines and feed ratio of IL (1)- Ethanol (2)- 1-butanol (3)- Water (4) atT=298.15 K andp=1 atm.
E/B and W/IL stand for feed molar ratio of ethanol to 1-butanol and water to IL respectively. Chapter 4: Simultaneous extraction of ethanol and butanol from aqueous solution102 area parameter (q) was taken as 2.11 and 1.97 respectively. For both quaternary systems, the coupling lines are predicted by NTRL and UNIQUAC with experimental coupling lines in Figs.
![Figure 4.1: Extract phase 1 H NMR spectra for the system: [TDTHP][Phosph] (1) - -Ethanol (2) - 1-Butanol (3) - Water (4) at T=298.15 K and p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/119.892.166.775.186.1044/figure-extract-spectra-tdthp-phosph-ethanol-butanol-water.webp)
Introduction
Chapter 5: Optimizing the Multistage Extractor 110 by solving material balance equations (M), phase equilibrium relation (E), mole fraction summation for each stage (S) and energy balance equations (H), better known as MESH equations. We have attempted to achieve the optimal number of stages and solvent flow rate by minimizing the multistage extractor cost for the extraction of butanol and ethanol from aqueous solution using ILs; [TDTHP][DCA] and [TDTHP][Phosph].
Computational details
Chapter 5: Multistage Extractor Optimization 114 For the upper (j=1) and lower (j=N) stages, the raffinate (R0) and extract (EN+1) flow rates are zero. Chapter 5: Multistage Extractor Optimization 115 where N and C represent the total number of trays and the total number of components, respectively. Chapter 5: Optimization of a Multistage Extractor 116 and the extract and raffinate flow rates leaving a particular tray.
Chapter 5: Optimizing Multistage Extractor 119 The position and velocity bounds were applied to the updated vectors to keep the particles within search space. Chapter 5: Optimizing Multistage Extractor 124 The penalty parameter by generation is updated based on tolerance (ε), whereε is a small positive number (=1). Chapter 5: Optimization of Multistage Extractor 125 the minimum sum of squared error for all particles is achieved.

Results and Discussions
Chapter 5: Multistage Extractor Optimization 131 Table 5.2: Parameter Tuning and Efficiency Analysis for [TDTHP][DCA] (1) - Ethanol. From table 5.4 it can be seen that the compositions are uniform in more than 6 steps. An attempt has been made to study the impact of IL costs on cost function values compared to other components.
This is bearing in mind that newer manufacturing technologies will reduce IL costs in the future. Fifty optimization runs were performed with reductions in IL costs ranging from 1/1 to 1/10,000 of current costs. Further reductions in IL costs (by 1000 and 10,000 times) reduced overall costs with the same butanol efficiency.
![Figure 5.5: Augmented Objective function (Eq. 5.33) Vs Generations for LD Inertia Weight for [TDTHP][DCA] (1) - Ethanol (2) - 1-Butanol (2) - Water (4) system at T =298.15 K and p=1 atm](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/152.892.113.729.203.1033/figure-augmented-objective-function-generations-inertia-ethanol-butanol.webp)
Research Conclusion
The NRTL and UNIQUAC models gave RMSD values less than unity for all systems, indicating a better fit. Ethanol efficiency was found to increase by a factor of nine when the cost of [TDTHP][Phosph] was reduced by a factor of 100. An additional 100-fold cost reduction for [TDTHP][DCA] indicates complete extraction (almost 100%) for both alcohols.
Future Scope
Liquid-liquid extraction of toluene from heptane using 1-alkyl-3-methylimidazolium-bis(trifluoromethylsulfonyl)imide ionic liquids. Effect of ammonium- and phosphonium-based ionic liquids on the separation of lactic acid by supported ionic liquid membranes (SILMs). Experiments, correlations and COSMO-RS predictions for the extraction of benzothiophene from n-hexane using imidazolium-based ionic liquids.
Volume, surface area, and UNIQUAC interaction parameters for imidazolium-based ionic liquids via the continuum polarization model. Screening of quantum chemical-based ionic liquids for the extraction of phenol from aqueous solution. Experimental and theoretical studies on the effectiveness of phosphonium-based ionic liquids for the removal of butanol at T=.
![Figure 2.4: Densities at different temperatures for [EMIM][MeSO 3 ] at p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/58.892.116.740.182.1031/figure-densities-different-temperatures-emim-meso-p-atm.webp)
![Figure 2.6: Densities at different temperatures for [EMIM][SCN] at p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/59.892.168.798.165.1087/figure-densities-different-temperatures-for-emim-scn-atm.webp)
![Figure 2.10: Densities at different temperatures for [TDTHP][DCA] at p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/61.892.172.794.348.853/figure-densities-different-temperatures-tdthp-dca-p-atm.webp)
![Figure 3.4: Raffinate phase 1 H NMR spectra for the system: [P 4441 ][MeSO 4 ] (1) - -Thiophene (2) - Cyclohexene (3) at T=298.15 K and p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/82.892.139.728.189.1048/figure-raffinate-phase-nmr-spectra-meso-thiophene-cyclohexene.webp)
![Figure 3.6: Experimental tie lines for the system:[TEMA][MeSO 4 ] (1) - Benzene (2) - Hexane (3) at T=298.15 K and p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/84.892.144.730.186.1052/figure-experimental-tie-lines-tema-meso-benzene-hexane.webp)
![Figure 3.11: Extract phase 1 H NMR spectra for the system: [TDTHP][DEC] (1) - -1-Butanol (2) - Water (3) at T =298.15 K and p=1 atm.](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10341670.0/91.892.174.775.190.1055/figure-extract-phase-nmr-spectra-tdthp-butanol-water.webp)