Carbon dioxide (CO2) and methane (CH4) are among the main contributors to the increasing global warming recently. Moreover, the reactivity of the catalysts is not only compared by their activation energies, but is more deeply explained by natural bond orbital (NBO), quantum theory of atoms in molecules (QTAIM) and fragment analyses. The activation of the CH bond via heterolytic cleavage is the rate-determining step with a free energy barrier of 27.6 kcal/mol at 298 K, in which the proton of CH4 is attracted to the ligand and the methyl anion is strongly attracted to the metal by become σ interaction with the dx2-y2 orbital of ruthenium.
Activation energies corrected for zero-point energies (ΔE0 K, in kcal/mol) and imaginary frequencies (IF, in cm–1) of the first and second transition states. Second-order stabilization energies (in kcal/mol) for interactions between natural bond orbitals of model complexes in the first transition state (in parentheses are the reactivity indices, in a.e., of the three model catalysts with the smallest first activation energy: µ represents the chemical potential, ƞ is the chemical hardness and ω is the global electrophilicity index.
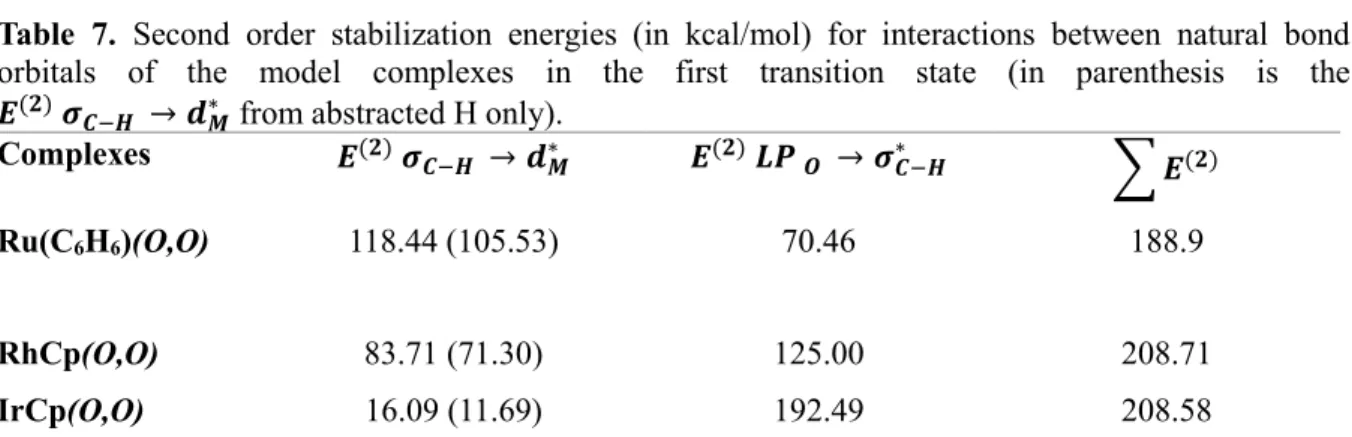
Second-order stabilization energies (in kcal/mol) for interactions between natural bonding orbitals 𝝈𝑪−𝑯 → 𝒅𝑴∗ of model complexes in the first transition with the corresponding Ej-Ei. Second-order stabilization energies (in kcal/mol) for the interactions between the natural bonding orbitals 𝑳𝑷 𝑶 → 𝝈𝑪−𝑯∗ of the model complexes in the first transition with the corresponding Ej-Ei.
Explanation of terms and abbreviations
INTRODUCTION
A combination of experimental and theoretical results in catalyst design has helped several studies optimize the electronic structure of the catalyst, resulting in high catalytic activity.3. Due to the inertness and non-polarity of carbon dioxide (CO2), the conversion to raw materials such as formic acid, methanol and acetic acid generally requires the use of higher temperatures due to the very unfavorable reactions.4 Therefore, a catalyst for the conversion processes is needed to accelerate the reaction processes. accelerate. Asymmetric hydrogenation is a reaction in which the hydrogen is preferentially added to either side of an unsaturated molecule, while transfer hydrogenation involves the use of an alternative hydrogen source such as isopropanol.
The metal center and nitrogen atoms of the diamine ligands are hydridophilic and protophilic species, respectively. The bifunctionality of Noyori complexes is an advantage in the design of catalysts for the conversion of stable CO2 gas into useful chemicals. There is a need to design a catalyst that can assist in the conversion of the two greenhouse gases, CH4 and CO2, into a raw material CH3COOH.
The possibility of the reaction mechanism would depend on the thermodynamic energies calculated using density functional theory. However, several problems with storage and transport of CO2 limit the binding of the said gas.14 Capture of CO2 via both weak non-covalent or strong covalent interactions limits its capacity for experimental and environmental conditions.15 CO2 storage and transport present problems with capacity and reservoir conundrum.16 The confusing pieces of CO2 post-processes led to the idea of practical and rapid CO2 conversion to natural raw materials such as acetic acid.
REVIEW OF RELATED LITERATURE
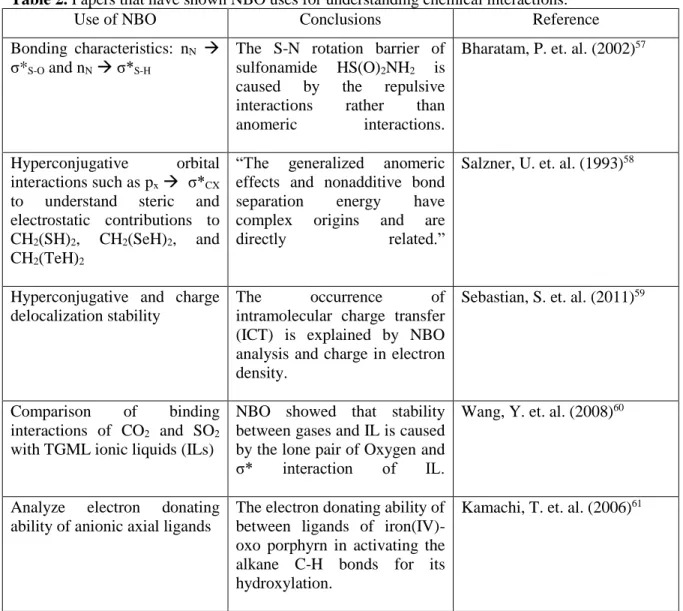
Coordination of the metal complex with the C-H bond is sometimes called pre-activation of the C-H bond. Phenanthroline and aromatic bipyridine increase the Lewis basicity of the ligand and metal center. Furthermore, catalyst recycling is still a challenge for most developed catalysts (with the exception of the Himeda work).
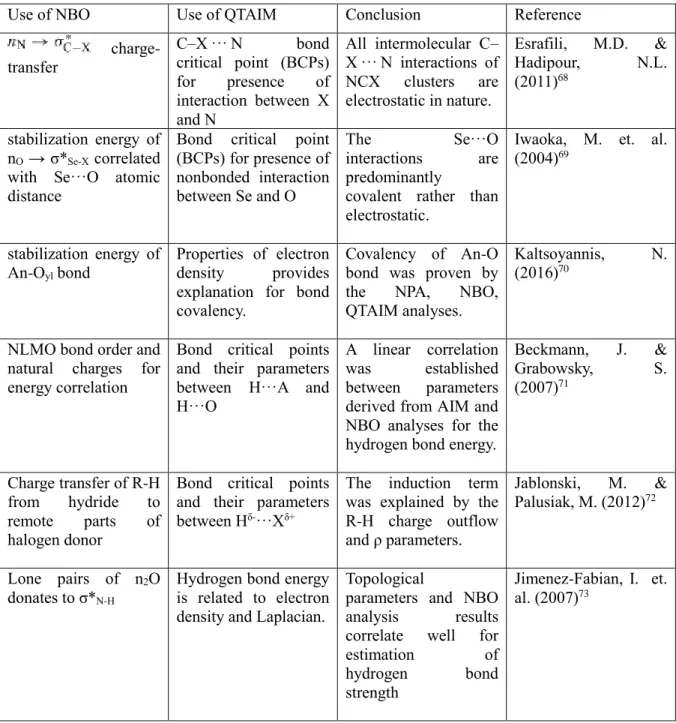
Zn/H-ZSM-5 zeolite CH4 and CO2 are co-transformed at 523-773 K due to the Bronsted acidity of the catalyst. Dominated by the attractive forces of the nuclei, the topology of the electron density has significant local maxima at the position of each nucleus. Laplacian of the electron density is attributed to the local curvature of ρ (r) and indicates whether the electron density is depleted, ∇2ρ (r) > 0, or locally concentrated, ∇2ρ (r) < 0 at a given point in the space.
The negative value of H(r) is attributed to some degree of covalent character of the interaction.66. This index can provide further details on the nature of the stabilization at the transition state of the reaction.
COMPUTATIONAL METHODOLOGY
Gibbs solvation energies were calculated by performing optimization calculations on implicit benzene and water based on all steady state structures obtained from the gas phase calculation to ensure that only one imaginary frequency is observed for the steady state structure. transition while there are no imaginary frequencies for the rest state structures. . Natural Bonding Orbital (NBO) charges of the atoms and second-order perturbation delocalization energy analysis were evaluated through the NBO calculation.80 All calculations were performed using the Gaussian09 suite of programs.81 Reactivity indices determined within DFT conceptual were calcd. The global electrophilicity index is defined as ω = (𝝁𝟐⁄𝟐ƞ), where μ is the chemical potential and ƞ is the chemical hardness.
In terms of one-electron energies of the frontier molecular orbital HOMO and LUMO, the two latter properties are expressed by 𝝁 = (𝜺𝑯𝑶𝑴𝑶+ 𝜺𝑳𝑼𝑴𝑶) 𝟐⁄❑ 𝜺𝑯𝑶𝑴𝑶).82Fragment analysis at the M06L/TZ2P level of theory was performed using from Amsterdam Density Functional (ADF) program package.83.
RESULTS AND DISCUSSION
Active catalyst models used in the calculations
- SUMMARY, CONCLUSIONS, RECOMMENDATIONS
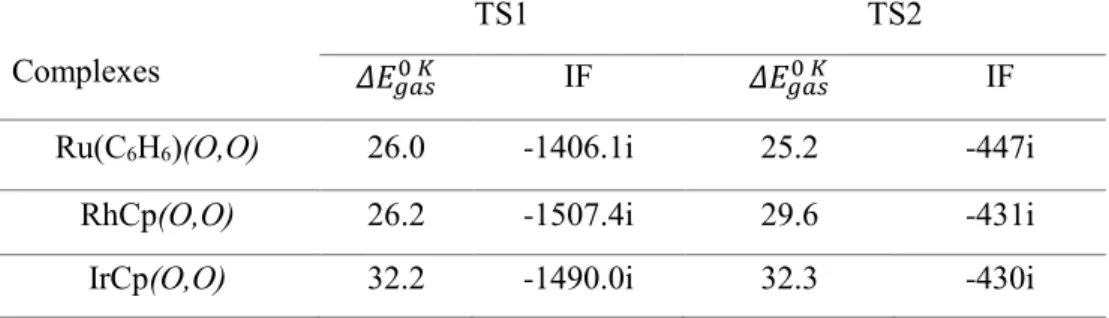
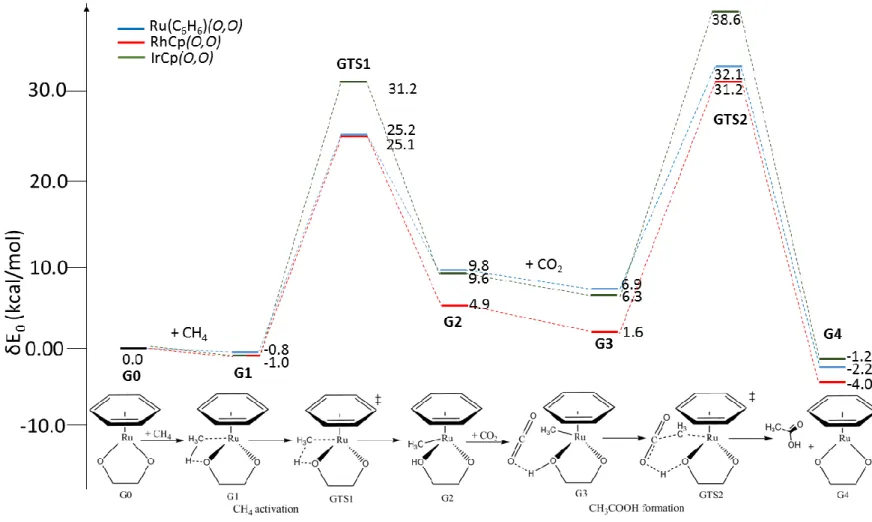
Activation and cleavage of the C-H bond through the 1st transition state of GTS1 produces a methyl complex (G2). Carbon dioxide is then added to interact with the protonated ligand, followed by concerted transfer of the methyl group and proton from the complex to CO2 (GTS2), forming acetic acid and regenerating the active catalyst (G4). Comparison of the energy profiles reveals that the lowest energy barriers are found for the Ru catalyst, with 26.0 kcal/mol for GTS1 and 25.2 kcal/mol for GTS2 (the acetic acid formation step).
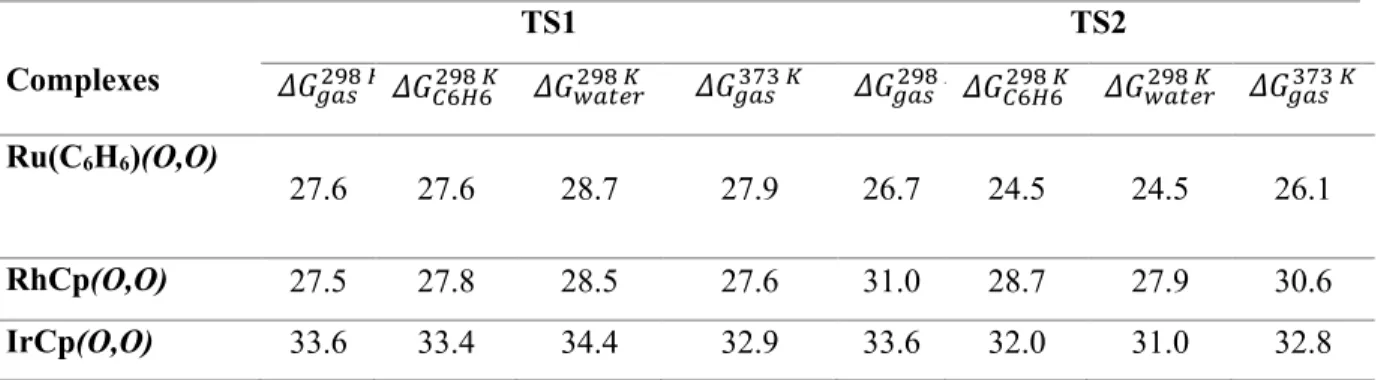
In the second transition state, CO2 interacts through its oxygen atom with the ligand proton (derived from CH4), while the carbon atom of the CO2 moves towards the carbon atom of the methyl group. For the Ru complex: the distance between the carbon atoms of the methyl group and CO2. The mechanism was calculated for reactions of the three complexes in the gas phase at 298 K and 373 K, as well as in benzene and water at 298 K (Table 6).
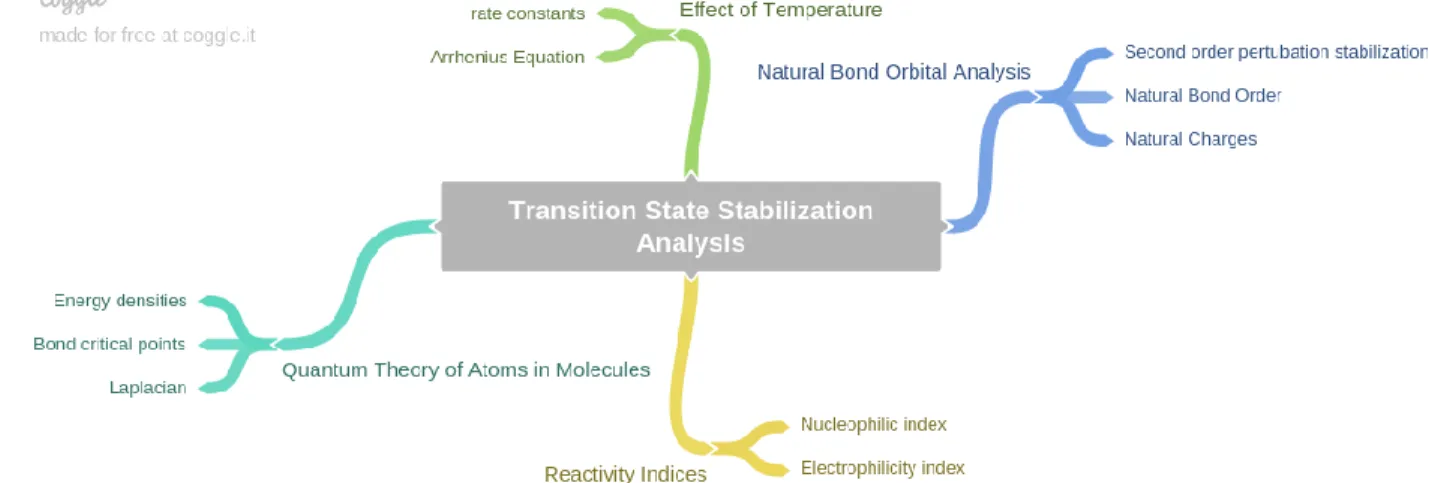
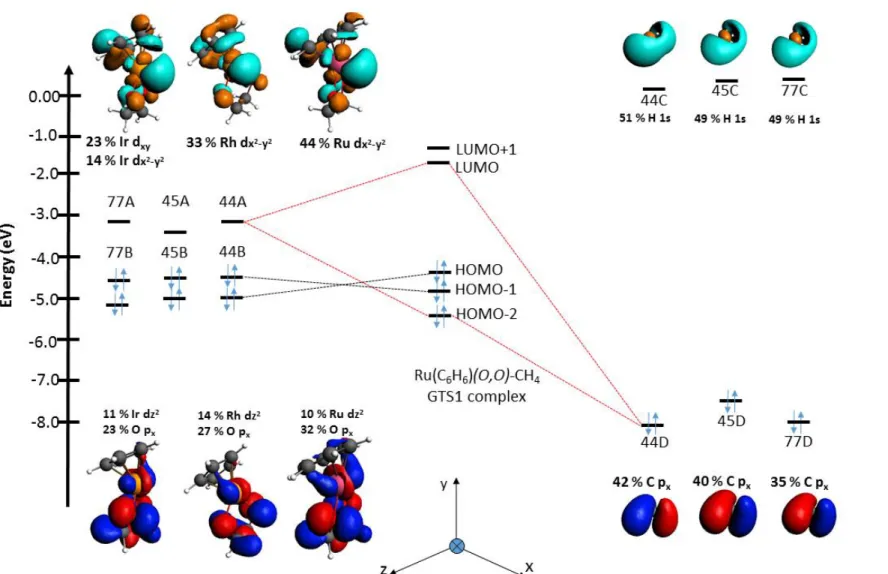
In particular, the calculated barriers show differences in the nature of the rate-determining step for the three catalysts. In this section, the interaction of the catalysts with the substrates in the transition states was investigated to gain insights into different activities. Fragment analysis of the interactions between the metal centers of the catalysts and methane was done to further investigate the orbital interactions (Figure 3).
No interaction between the HOMO of the catalyst fragment and the LUMO of the CH4 fragment was observed for any of the three cases. Orbital contributions of frontier molecular orbitals of the two fragments to the GTS1 state for different metals. This is indicated by the presence of a C-C-σ-bond orbital involving the carbon atoms of the methyl group and CO2.
In addition, a fragment analysis shows an interaction between the HOMO of the methyl group and the LUMO of CO2 for each of the three complexes. The rest of the ligands have > 2 kcal/mol difference from (N,NTs) in their 1st TS. Orbital contributions from frontier molecular orbitals of the two fragments at the GTS1 state for different ligands.
To explain the trend of activation energies of model catalysts, stabilization energies and charges of secondary natural bond orbital disorder (NBO), fragment analysis, quantum theory of atoms in molecules (QTAIM) calculations and reactivity indices were performed. In addition, there must be an equilibrium between the M-CH4 and CH4-CO2 interaction for the co-transformation to take place.
APPENDIX
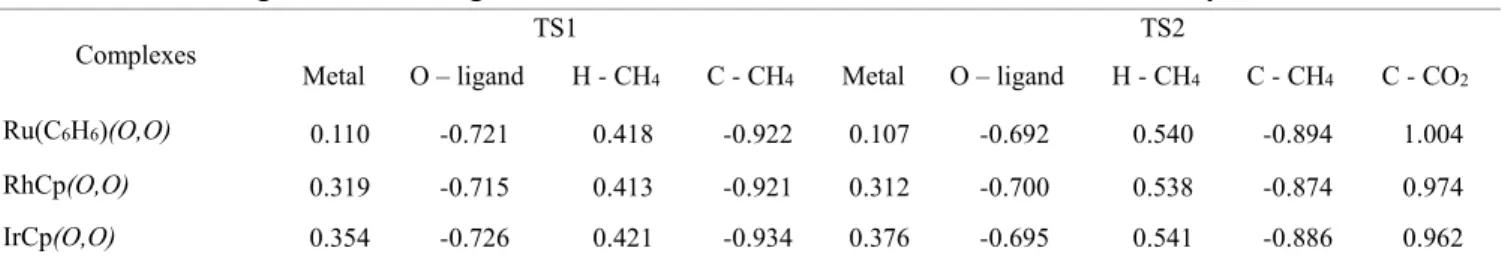
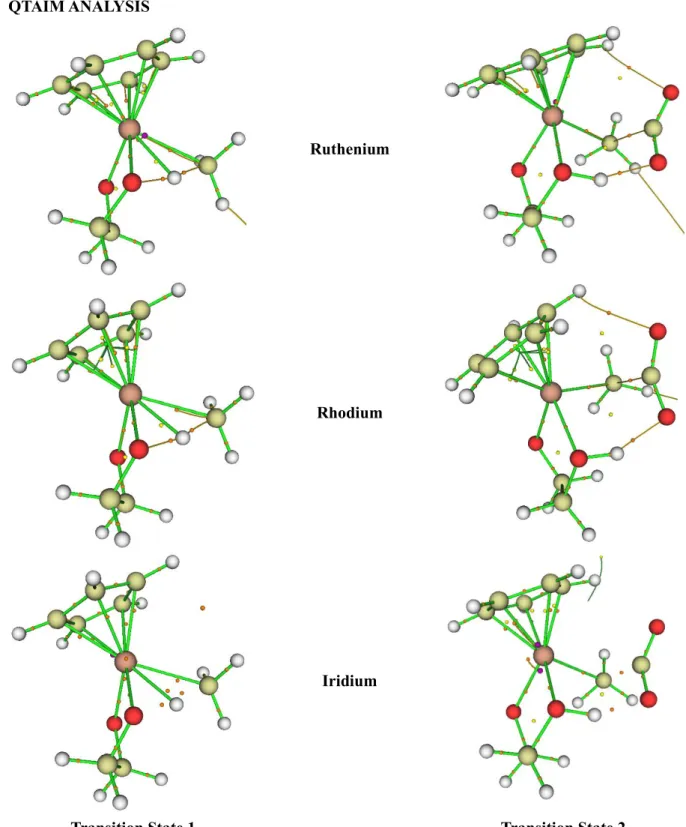
Molecular graphs of TSs for different metal centers analyzed in this study; large circles correspond to attractors (C: yellow; H: white; O: red; Metal: peach), small ones to critical points (orange: bond critical point ((3, -1) BCP); yellow: ring critical point ( ( 3,+1) RCP); green: cage critical point ((3,+3) CCP)). Molecular graphs of TSs for different ligands analyzed in this study; large circles correspond to attractors (C: yellow; H: white; O: red; Metal: peach; N: purple; S; yellow), small ones to critical points (orange: bond critical point ((3, -1) BCP ); yellow: ring critical point ((3,+1) RCP); green: cage critical point ((3,+3) CCP)). Topological parameters (in atomic units) for M…CH3 (3,-1) critical points for 1st transition state with varying metal centers.
Topological parameters (in atomic units) for different (3,-1) critical points for the 2nd transition state with different metal centers. Topological parameters (in atomic units) for M…CH3 (3,-1) critical points for the 1st transition state with different ligands. Topological parameters (in atomic units) for different (3,-1) critical points for the 2nd transition state with different ligands.
ACKNOWLEDGMENT