This thesis describes the research performed on the 'Development of the Visible Light Photoredox Catalyzed Reactions Using Organo-silane reagents'. It also provides a brief overview of the silicon reagent, its abundance and stabilization, role in radical generation and photochemical methods based on organosilicon reactions.
Introduction
- Introduction
- Instrument setup
- Mechanism
- Rehn-Weller Equation
- Importance of Photoredox catalysis
- Generation of stabilized radicals by photoredox catalysis
- Importance of Silicon in organic synthesis
- Silicon-based TAGs
- α-Amino silane
- α-Silyl Ether
- Hydroxymethylsilane
- α-Thio Silane
- Acyl silane
- References
General ways of generating radicals by homolytic cleavage of C-H bonds at a heteroatom (Scheme-1.2a). The silyl group is then cleaved off, resulting in the formation of an alkoxymethyl radical that can contribute to the diversity of electrophiles.
Visible-Light Photoredox-Catalyzed α-Regioselective Conjugate Addition of Allyl
Abstract
Introduction
The implication of this result highlights the use of the silane reagents for the successful generation of the respective radicals and their addition to the alkenes. With this in mind, we used the allylsilane reagent to generate the allyl radical and then bind it to the electron-deficient alkenes.
Results and Discussion
- Optimization
- Substrate Scope
- Applications
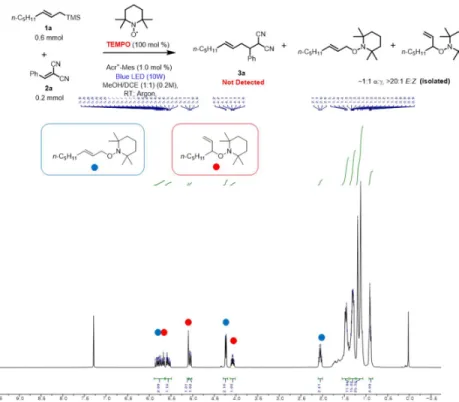
We performed a control experiment in the absence of photocatalyst and light to check the dependence of the reaction on both parameters. Arylidenemalononitriles containing disubstituted and trisubstituted arenes also underwent the reaction and gave the desired product in moderate to good yields with the exclusive formation of the α-adduct.
![Table 2.1 1. Optimization of the reaction conditions. [a]](https://thumb-ap.123doks.com/thumbv2/123dokinfo/10355894.0/31.918.146.779.288.666/table-optimization-reaction-conditions.webp)
Mechanistic Investigation
- Cyclic Voltammetry
- Fluorescence Quenching experiment
- On-Off Experiment
- Radical trapping experiment
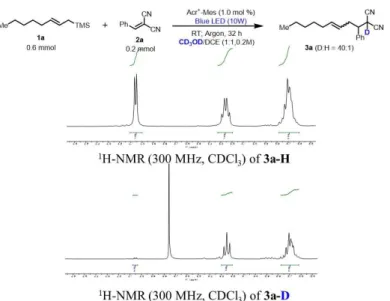
- Deuterium labeling Experiment
In addition, Stern-Volmer fluorescence quenching experiments were performed with allylsilane 1a and benzalmalononitrile 2a (Figures 2.1 and 2.2). This result shows that a SET mechanism between allylsilane 1a and the photocatalyst occurs under blue LEDs irradiation.

Mechanism
Conclusion
Supporting Information
Following the general procedure using 5% ethyl acetate in hexanes as eluent, 3k was obtained as a colorless liquid (49.1 mg, 83% yield,);. Following the general procedure using 5% ethyl acetate in hexanes as eluent, 3r was obtained as a colorless liquid (32.7 mg, 63 % yield,);.
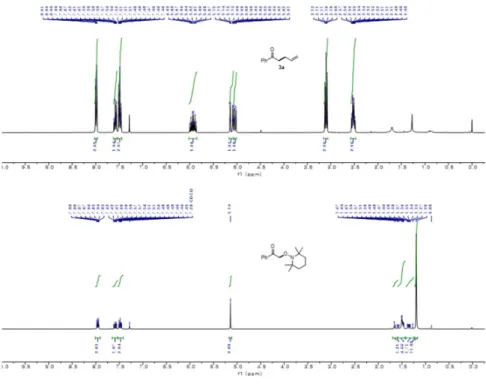
H and 13 C NMR Spectra
Visible-Light Photoredox-Catalyzed α-Allylation of α-Bromo-carbonyl Compounds
Abstract
Introduction
Pd-catalyzed allylation of nucleophiles with allyl halides, acetates and carbonates (Tsuji-Trost reaction) has been shown to be a mild method for the α-allylation of carbonyl compounds 5,8-10, which is widely used due to its mild reaction conditions. wide substrate range and stereochemical control.(Scheme-3.2) However, this reaction is limited only to stabilized enolates such as an anion of malonate and carbon nucleophiles, whereas enolates of ketones, esters and amides are rarely used. Reiser and co-workers explored the potential use of allyltributyltin as an allylation reagent for the α-halocarbonyl compounds using the copper phenanthroline photocatalyst (Scheme-3.4)24. Allyl trifluoroborate is a new allylation reagent and its use for allylation of the α-halocarbonyl group shown by Yasuda and co-workers.
Reiser and co-workers reported 24 allyltrimethylsilane as an allylation reagent in photoredox-catalyzed α-allylation of carbonyl. Our research group is primarily interested in the use of organosilicon groups in photoredox catalysis, and we have recently discovered that organosilicon groups can act as excellent radical precursors in photocatalytic reactions.26-28 In view of the development of the non-toxic and greener reagent for allylation reaction, we also considered the possibility of using allyltrimethylsilane as a new allylation reagent for α-allylation of carbonyl compounds because it is neutral, stable and non-toxic. The visible light photoredox-catalyzed α-allylation of various carbonyl compounds using allyltrimethylsilane is described here.
Result and Discussion
- Optimization
- Substrate Scope
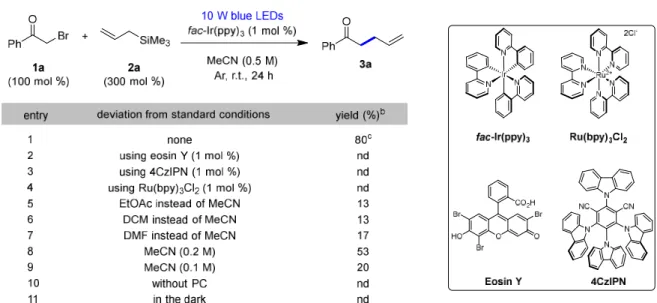
The control experiments showed that the photoredox catalyst with visible light was crucial in the reaction (Table 3.1, points 10-11). We investigated the scope of α-allylation of carbonyl compounds using different α-halocarbonyl compounds with optimized reaction conditions (Table 2). In addition, the results showed no relationship between the electronic environment of the ketone and product yield.
Moreover, the substrate scope was successfully extended to α-bromoesters ( 1i , j ) and α-bromoamide ( 1k ), and the esters and amides reacted similarly to ketones. Substrate investigations showed that α-allylation of ketones, esters and amides was efficient in photoredox catalysis. We predicted the mechanism for the reduction of 2-bromomethophenone 1a and the α-carbonyl radical was generated by an excited photocatalyst.
Mechanism
We proposed the reaction mechanism depicted in Scheme 2b based on our control experiments and literature reports 20,31-33 The photocatalyst fac-Ir(ppy)3 was excited by irradiating it to blue LED and the excited photocatalyst reduces 2-bromoacetophenone 1a . After that, Br- was released from the reduced 1a, resulting in the formation of the α-carbonyl radical intermediate I. Radical addition of an electrophilic α-carbonyl radical I and an electron-rich alkene 2a gives 3a via radical elimination trimethylsilyl.
Mechanistic studies
- Luminescence quenching Study
- Radical trapping experiment
The steady decrease of the emission intensity of the fac-Ir(ppy)3 solution with the gradual increase of the amount of 1a as presented in (Figure 3.1a and Figure 3.1b) supports that the reaction mechanism occurs through the oxidative quenching cycle. To investigate the nature of the reaction, the controlled reaction was performed with radical scavenger (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) Scheme 3.7. The desired product was not observed, but TEMPO addition products were observed instead, suggesting that the reaction involved an α-carbonyl radical intermediate.
Conclusion
General procedure and characterization data of products
Divergent synthesis of Amino alcohols
Abstract
Introduction
Although their process uses cheap and readily available starting materials and provides access to the nearby amino alcohol in a single step, harsh oxidizing reagents and the use of a substoichiometric metal reagent are necessary for the synthesis of 1,2 amino alcohols. This amino acid, after decarboxylation, forms the amino radical, which then intervenes with the aldehyde to form the adjacent amino alcohol products. Subsequent oxidation and hydrolysis of this intermediate yields the amino alcohol with primary alcohol group.
Despite this, all the reactions of amino alcohol synthesis mentioned here are of 1,2 amino alcohol with a secondary alcohol group. The primary alcohol group in the 1,2-amino alcohol moiety enables direct synthesis of amino acids through oxidation. We discovered the amino alcohol synthesis while studying the use of silicon reagents in various reactions.
Result and Discussion-
- Optimization
- Substrate Scope
We assumed that the cleavage of the PMP ether was caused by oxygen in degassed MeOH. To increase the reaction yield, we considered the addition of the oxidizing state in the following step, i.e., after the coupling reaction is completed. Later, aromatic imines containing photolabile functional groups were tried, e.g. halogens such as bromo, chloro and fluoro substrates and these substrates were well tolerated in the reaction and gave excellent yields of the β amino ether and the corresponding amino alcohol products.
Ortho-, meta- and para-substituted chloroaldimines gave the best yields of the β-amino ether and amino alcohols (3g-3i and 4g-4i), suggesting that this reaction is not mediated by the position of the chlorine group on the aromatic aldimines is not affected. Furthermore, in the case of the para-substituted aromatic aldimines, almost quantitative yields of β-amino ethers with the 4CzIPN catalyst, thus emphasizing the orthogonal nature of the reaction (3i). Therefore, the preparation of the fluorine-containing compounds in addition to the amino alcohol is of crucial importance.
Applications
- Reaction in sunlight
- Scale-up
- Product Diversification
When we tried the aromatic aldimines containing fluorine, the resulting β-amino ether and amino alcohol were obtained in excellent yields (3j and 4j). When heterocyclic aldimines were tested in the reaction conditions, corresponding β-amino ether products were obtained in the best yields (3k, 3l). Unfortunately, the resulting deprotection reaction of these β-amino ether products was unstable and decomposed in the reaction conditions.
Complex and bulky aromatic aldimines such as disubstituted and trisubstituted aromatic aldimines were then tested and able to smoothly provide the resulting coupling and subsequent deprotection product (3m,3n, 3o and 4m,4n,4o). Aldimines derived from the methanesulfonamide, and the benzenesulfonamide have also been well worked up and able to give the corresponding coupled and deprotected product in excellent yield. The conversion of 4a into aziridine, a useful synthetic intermediate, was accomplished in a single step with a 67% yield (Scheme 4.14, 5a) The second application of 4a products was realized by converting to oxazolidines, which are a essential role in ligand synthesis (Scheme 4.14, 5b and 5c).
Mechanistic Investigation
- Fluorescence Quenching-
- Cyclic Voltammetry
- Radical trapping experiment
To verify the radical nature of the reaction, radical scavenging reactions with TEMPO were performed. When the coupling reaction of imine 1a and 2a was performed in the presence of TEMPO, as expected, no coupling products were observed under both Ir and 4CzIPN catalytic conditions, demonstrating the radical nature of the reaction (Scheme 4.15). Also to verify the radical nature of the deprotection reaction, 3a was exposed to the oxidative state with Ir catalyst and 4CzIPN in the presence of TEMPO.
In both cases, no deprotected product 4a was observed, proving that the reaction follows the radical mechanism (Scheme 4.16).
Mechanism
Excited photocatalyst oxidizes the 3a and forms the aryl cation radical species IV and photocatalyst is reduced Ir-1. This reduced photocatalyst is then oxidized by the molecular oxygen from the air 1, thus leading to the formation of superoxide anion radical 2, and photocatalyst reaches the ground state Ir*. Superoxide anion radical 2 attacks the aryl cation radical IV and leads to the formation of aromatic cationic species V.
This cationic species V is believed to undergo nucleophilic attack by the methyl peroxide anion 3, resulting in the formation of the hydroperoxide species VI.
Conclusion
The resulting materials were converted into a variety of valuable synthetic intermediates, including synthetically valuable amino acids.
Supplementary Information
- References
After the completion of the reaction, the solvent was removed under reduced pressure and the residue was purified by flash column chromatography on silica gel to give the corresponding amino alcohol products 4, dtbpy)]PF6 and 4CzIPN, respectively. After completion of the indicated time, the solvent was removed under reduced pressure and the residue was purified by flash column chromatography on silica gel to give the corresponding product 3a (1.51 g, 84%). First of all, I would like to convey my heartfelt appreciation to my mentor and advisor, Prof.
My sincere gratitude goes to my former labmates and friends, Kim Kyung Tae, Kim Myeoung Jun, Zhang Gwang Seok, Ahn Deok Hyun, Nam Subeen, Kim Suhyeon, and Kang Young Woo. I would like to express my gratitude to my current labmates, Park Jin, Kim Ran hee, Heo Hyun Huh, Shafrizal, Kim Yejin and Park Sanghyeon. Big thanks to my dear friend Tanvi Nair for the encouragement and assistance in improving English written skills.
I am very grateful to my cousin brothers and sisters, and every dear family member for their love and care. I would like to thank my beautiful son Any, who has brought so much joy to my life.