I am very grateful to all the professors in the Department of Civil and Environmental Engineering. The novelty of this research work lies primarily in the utilization of waste foundry sand to partially replace fine aggregate in GPC. Furthermore, cured GPC samples achieved heavy metal immobilization in the range of 98–100%, which is promising for environmental applications.
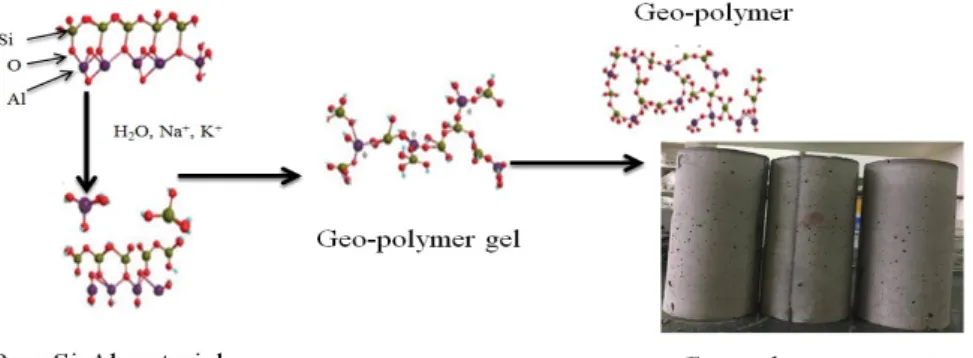
The alkaline solution used for GPC converted the heavy metal compounds into highly insoluble metal hydroxide materials and at the same time encapsulated the thermal waste ash.
INTRODUCTION
Background of study
Portland cement is most widely used in the construction sector, and during the year 2016 alone, almost 4200 million tons of cement were produced globally. Based on the cement consumption and production for the year 2016 worldwide, China produced 2480 million tons, while India produced 290 million tons of cement. So in this study Portland cement used low calcium fly ash (LCFA) and ground granulated blast furnace slag (GGBFS) was used in geopolymer concrete.
However, geo-polymer cement is a new type of cement that uses a different chemistry than that found in traditional OPC.

Objective and scope
Thesis structure
LITERATURE REVIEW
- Ordinary Portland cement (OPC)
- Geo-polymer concrete (GPC)
- Geo-polymerization process
- Mechanism of heavy metal immobilization
- Foundry sand replacement in concrete review
- Key components of GPC
- Fly ash
- Ground granulated blast furnace slag (GGBFS)
- Waste foundry sand
- Heavy metals in geo-polymer concrete
- Lead (Pb)
- Chromium (Cr)
- Copper (Cu)
- Zinc (Zn)
- Nickel (Ni)
- Iron (Fe)
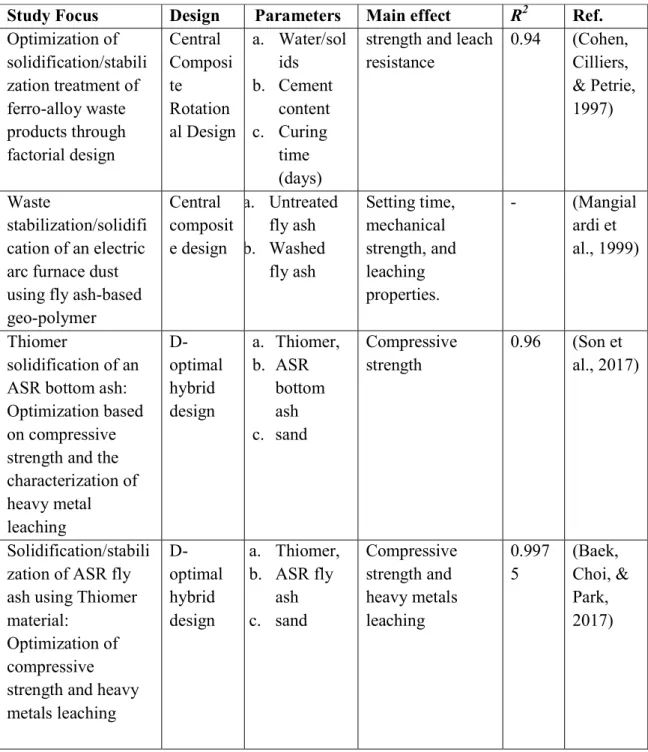
- D-Optimization
The compressive strength decreased with increasing replacement of silicate-bound sand and clay-bound sand.. 19 . further demonstrate the use of waste foundry sand as a partial replacement for fine aggregate in concrete. Through the experimental results, they conclude that the compressive strength increases with the increase in the fractional replacement of foundry sand waste and the split tensile strength decreases with the increase in the percentage of foundry sand waste. The compression value of GPC depends on the time and type of curing, i.e., the age of hardening and the temperature to which the molds are subjected.
The test results showed a marginal increase in the strength properties of plain concrete by incorporating waste foundry sand as a partial replacement of fine aggregate. Waste foundry sand is a major byproduct of the metal casting industry and has been used successfully as a backfill material for many years. But the use of foundry sand waste for land filling is becoming a problem due to the rapidly increasing cost of its disposal.
MATERIALS AND METHODS
- Materials
- Cement
- Fly ash
- GGBFS
- Foundry sand
- Fine aggregate
- Coarse aggregate
- Alkaline solution
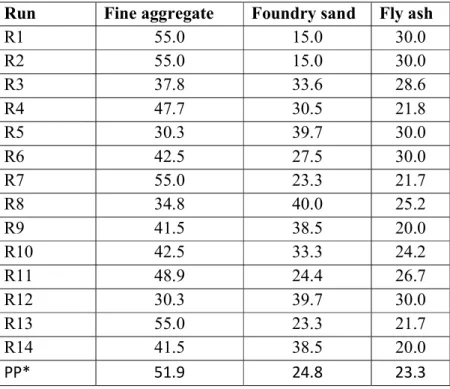
- Statistical experimental mixture design
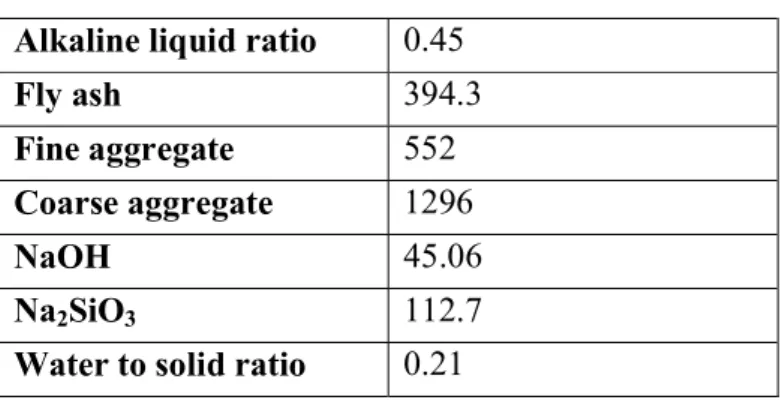
- Mixture design calculation of GPC
- OPC and GPC Specimen
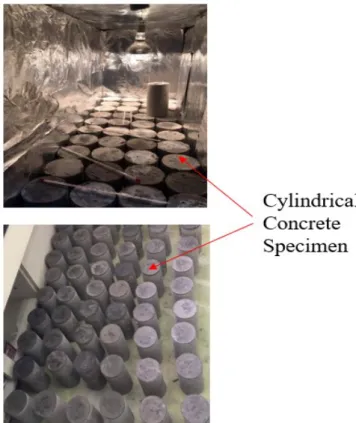
- Specimen preparation
- Curing
- Physico-chemical characterization
- Compressive strength
- Crystal structure and chemical characterization
- Determination of heavy metal content in incineration fly ash
- Heavy metal leaching evaluation
GGBFS which is also used to replace the cement and the specific gravity of GGBFS is 2.97. Waste foundry sand was used to replace as fine aggregate, and the specific gravity of foundry sand is 2.71. In the current study, the available river sand near Ulsan has been used as fine aggregate.
Locally available blue granite crushed aggregate of 12.5 mm size was used in this investigation and the samples were subjected to two tests by screening analysis and specific gravity test. In this study, we used a combination of sodium hydroxide (NaOH) and sodium silicate (Na2SiO3) solutions. The sodium hydroxide solution was prepared by dissolving flakes or pellets in transfer water.
The mass of sodium hydroxide solution is modified depending on the concentration of the solution transferred in terms of molarity (M). Using the D-optimal design software, it can determine the optimal combination of the three materials in the mix. After 24 hours, the GPC sample was taken from room temperature and the sample was reformed.
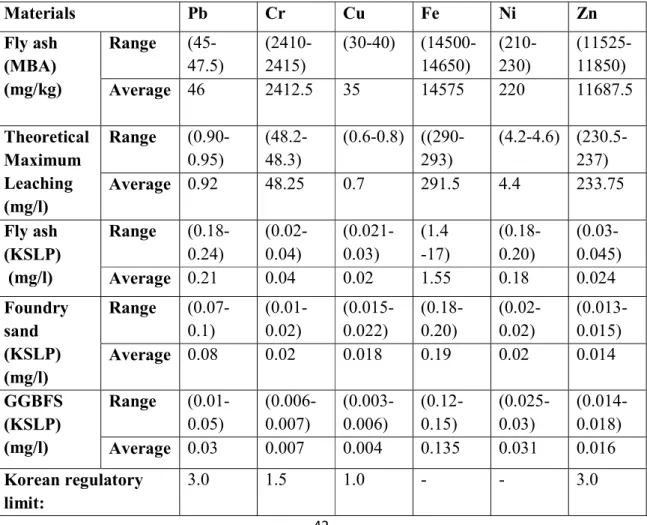
An atomic absorption spectroscopy (AAS) was used to measure the full concentration of heavy metals in the state of acidic solutions. To understand the mobility of the heavy metals of the above solidification, the Korean Standard Leaching Procedure (KSLP) method (Osada, Tanigaki, Takahashi, . & Sakai, 2008; Son et al., 2017) was performed in this study and compared the results.
EXPERIMENTAL RESULTS AND DISCUSSION
Heavy metal contents and leaching concentrations
4-1 shows the GPC coagulation of the 14 runs after heavy metal leaching concentration under the KSLP method in different mixing ratios of foundry sand, fly ash and GGBFS. Moreover, the heavy metal concentration in the mixture of fly ash, foundry sand and GGBFS materials has the highest leaching capacity in the order of Cu and Cr > Pb > Zn > Fe > Ni. The highest leaching concentration in Pb is R2 (0.2mg/l), this is attributed to a higher content of foundry sand which leads to low leaching results in Pb.
The lowest concentration of leachate in Pb is in R10 (0.17mg/l) this is due to a higher mixing ratio of foundry sand. A low Cr leaching result is observed when fly ash decreases and foundry sand increases. Cu leaching result in R4, R5 and R13 have the highest leaching concentration due to more foundry sand and less ash ratios.
When the fly ash ratio becomes lower and foundry sand higher, the Ni leach concentration is lowered. In addition, when the foundry sand and fly ratio is in the average interval, the Zn leaching concentration is reduced, as seen in R7 and R13. Based on the overall results, the increase in foundry sand content lowers the leaching concentration of heavy metals.
This is due to the presence of silica in foundry sand, as reported in Siddique & Noumowe (2008). Based on the overall results of heavy metal leaching, the addition of GGBFS with foundry sand and fly ash can potentially reduce heavy metal leaching from the concrete in any proportion.
Compressive strength
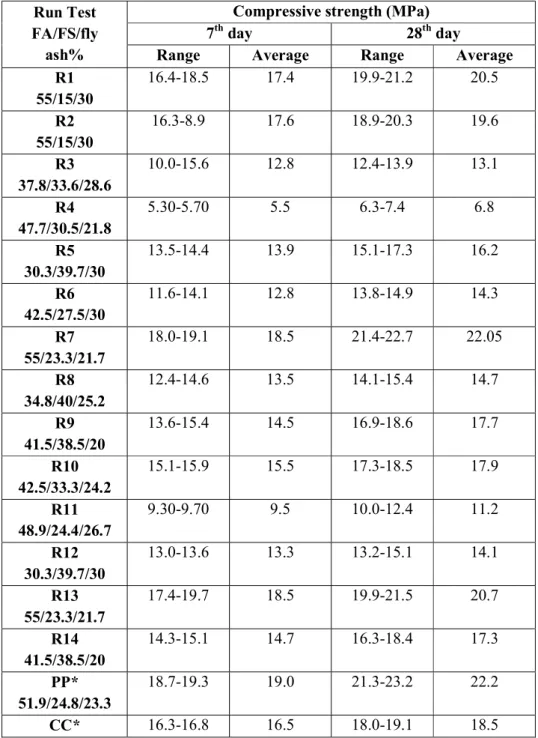
The minimum compressive strength of GPC is R4 5.5Mpa (7th day) and 6.8Mpa (28th day), and the maximum compressive strength is 19.0Mpa (7th day) and 22.2Mpa (28th day), but the strength of conventional concrete 16.5Mpa (7th day) and 18.5Mpa (28th day). The strength of conventional concrete is lower than compared to GPC, due to waste foundry slag, fly ash and alkaline solution. Waste foundry sand, fly ash and alkaline solution have a higher content of silica, so their property of quartz is increased strength of concrete. If you add more foundry sand and fly ash, this time the compressive strength decreases because the silica properties increase and the CaO decreases.
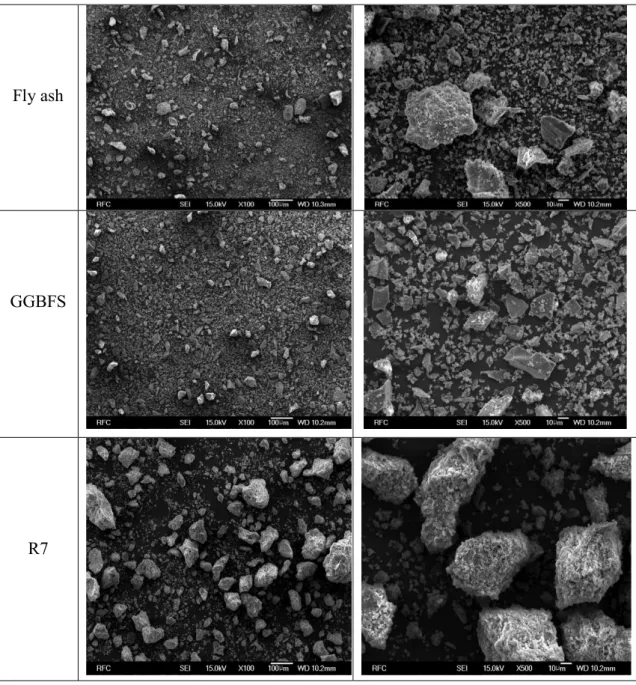
FE-SEM &XRD analysis
Through XRD analysis, it is observed that the composition and pattern of ash containing many harmful substances are immobilized. Therefore, reuse and recycling of fly ash materials can be encouraged in building materials. The main components of fly ash material (before use) mainly consist of quartz (SiO2), in addition to other minor components such as calcium oxide (CaCO3), hematite (Fe2O3), aluminum oxide hydrate (5A12O3-H2O), dolomite CaMg(CO3)2), calcium silicate hydrate (Ca1.5SiO3.5.nH2O) and calcium aluminate hydrate (Ca3Al2O6.nH2O) (Swanson & Tatge, 1951).
When most of the fly ash crystallizes in the form of the hydrate, all of the aforementioned metals have been converted to sulfides/sulfites. As a result, it appears that the coagulation of harmful substances (heavy metals) by fly ash is effective.
Response surface optimization
Therefore, solidification of heavy metal compounds when mixed with GPC is believed to be caused by metal hydroxide formed by chemical reaction. The response coefficients (βi and βij) for the independent variables (A, B and C) were determined using the preliminary data.
Statistical results
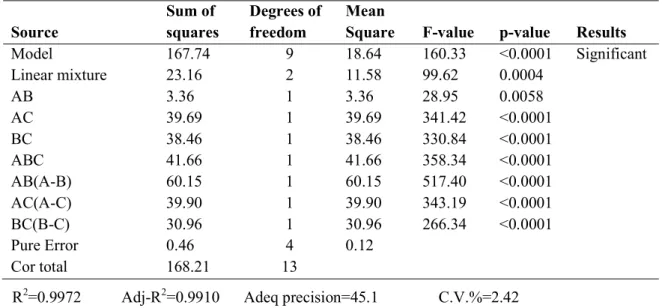
At 7 and 28 days, the interactive terms of AB, AC, and BC were significant in the compressive strength in solidifying GPC. In the mathematical model, the usually reliable adjustment is based on the coefficient of the drive (R2). The adjusted coefficient of the drive (Adj-R2) is generally used to define the important fit quality.
The high Adj-R2 for both compressive strengths confirmed that the generated cubic model can determine the exact range of the tested model and provides a good fit. This indicates that some of the fit of the model can be used to optimize its appropriate responses. The signal-to-noise ratio determines the adequacy of the signal that can be observed at each value of the appropriate precision.
Consequently, they obtained a ratio of 7 to 28 days of compressive strength (45.304 and 18.945) for the Adeq accuracy in the quadratic model implying sufficient signal to drive the design space. The attribute of precision, where strategies are associated, is judged by the coefficient of variation (C.V.%). For low C.V. Based on the formed quadratic model, Figs and 4-9 show the characteristic plots of the GPC clotting process.
It can be seen that 7th and 28th day compressive strengths are high, based on Fig. 4-8, and 4-9, the interior residuals showed that the data points that are close to normal line have an adequate fit for the model.
Analysis of responses
Implication of the results
Conclusion
The total concentration of heavy metals in fly ash is estimated by the MBA method. It also shows that GGBFS; the combination of waste foundry sand and fly ash can potentially reduce leaching of heavy metals. As a result of this study, it is confirmed that the properties according to the mixing ratio of fine aggregate, waste foundry sand, fly ash can be effectively used as an environmentally friendly building material by increasing the aspect of added value through recycling and reuse of by-product sources.
However, before its use, combustion fly ash containing many harmful substances can be subjected to possible leaching. Only a partial replacement of fine aggregate with foundry sand is currently investigated. Therefore, future work is recommended for complete replacement of fine aggregate and improvement of immobilization efficiency.
Microstructure and properties of concrete using fly ash and waste foundry sand as partial replacement of fine aggregates. Reducing the water demand of air-entrained concrete containing large amounts of fly ash. Experimental investigation of the use of fly ash and waste foundry sand as a partial replacement material in concrete.
Comparative investigation into the influence of used foundry sand as partial replacement of fine aggregates on the properties of two grades of concrete. Optimization of Transesterification of Biodiesel Using Green Catalyst Derived from AlbiziaLebbeck Pods by Mixture Design.