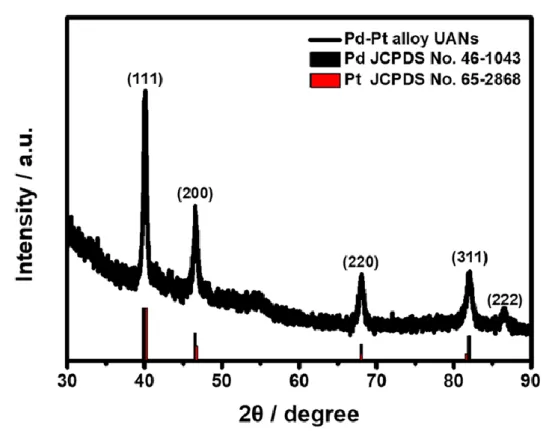
금속촉매의 활성을 높이는 방법으로는 형상제어, 조성제어, 크기제어 등이 있다. 본 연구의 목적은 알카리성 용매에서 에탄올 산화 반응에서 촉매의 모양과 조성을 제어하여 활성을 증가시키는 것이다. 이 연구는 팔라듐-백금 합금을 입자 주위에서 시트가 성장하는 계층적 블룸 형태로 제시함으로써 활성을 강화했습니다.
적층된 Pd-Pt 나노시트의 계층적 블루밍 형태는 주사전자현미경과 투과전자현미경으로 확인하였다. KI, CTAC 및 AA는 계층적 개화 형태를 형성하는 중요한 요소입니다. 시약의 특성을 이용하여 동일한 조성의 Pd-Pt 나노결정(NCs)을 모양에 대한 대조군으로 합성하였다.
이 실험을 통해 촉매의 형태와 조성이 전기화학적 특성에 영향을 미치는 중요한 인자임을 확인하였다. 그들의 계층적 형태의 Pd-Pt 나노결정은 주사전자현미경(SEM)과 투과전자현미경(TEM)으로 확인되었다. 또한 동일한 조건에서 300 사이클의 CV 후에도 Pd-Pt 초박형 조립 나노시트의 질량 활성은 70% 이상을 유지했습니다.
Herein, we report the appropriate synthesis method to form Pd-Pt ultrathin assembled nanosheets (UANs) with low-coordinate and Pd-Pt alloy surfaces.
Experimental
Result and Discussion
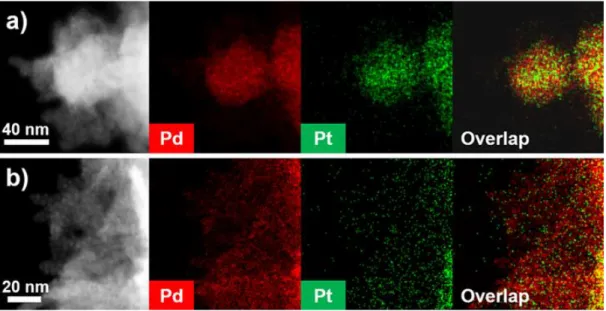
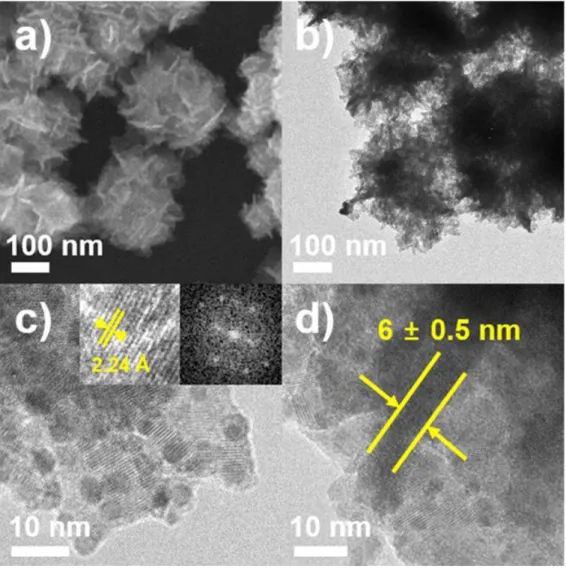
Insets in Figure 1c show high-magnification HRTEM image and FFT pattern obtained from an ultrathin nanosheet subunit of Pd-Pt UAN. d) HRTEM image of the side surface of an ultrathin nanosheet subunit. The composite structure of the ultrathin assembled nanosheet (UAN) was measured by elemental mapping with HAADF-STEM energy-dispersive X-ray spectroscopy (HAADF-STEM-EDS), which clearly confirmed the formation of a homogeneous Pd-Pt alloy structure. The Pd/Pt atomic ratio of the ultrathin assembled nanosheet studied by inductively coupled plasma-optical emission spectroscopy (ICP-OES) was about 10:1).
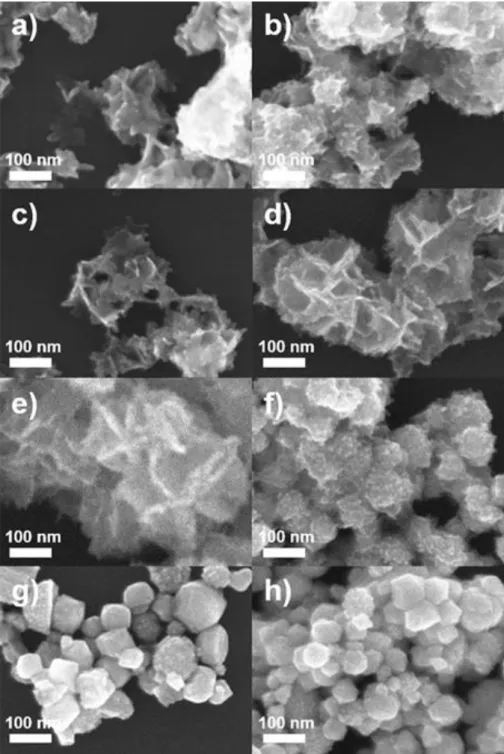
It can be interpreted that the single crystals of 14.52 nm form Pd-Pt ultrathin composite nanosheets (UAN). For the synthesis of ultrathin Pd−Pt composite nanosheets (UAN), tuning the growth behavior of the nanostructures by controlling the amount of I-ions, AA, and CTAC concentration is a critical factor. Further, to investigate the influence of CTAC used as a surfactant to form Pd−Pt ultrathin composite nanolayers (UAN), a control experiment was conducted using different amounts of CTAC (Figures 5).
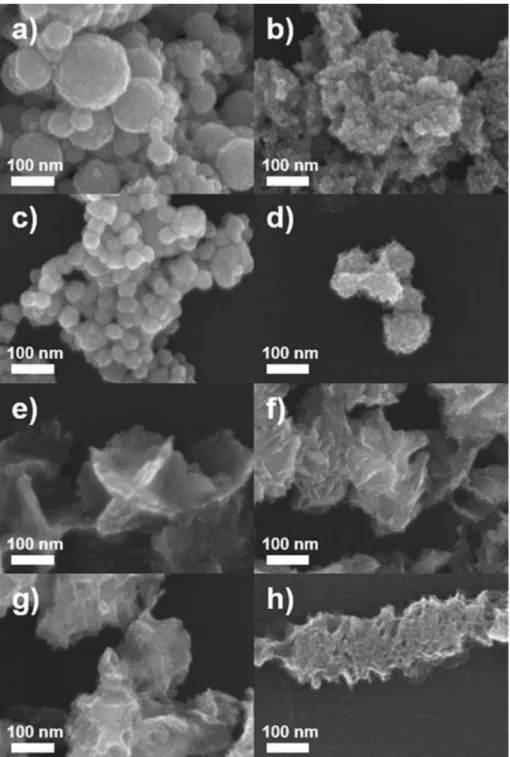
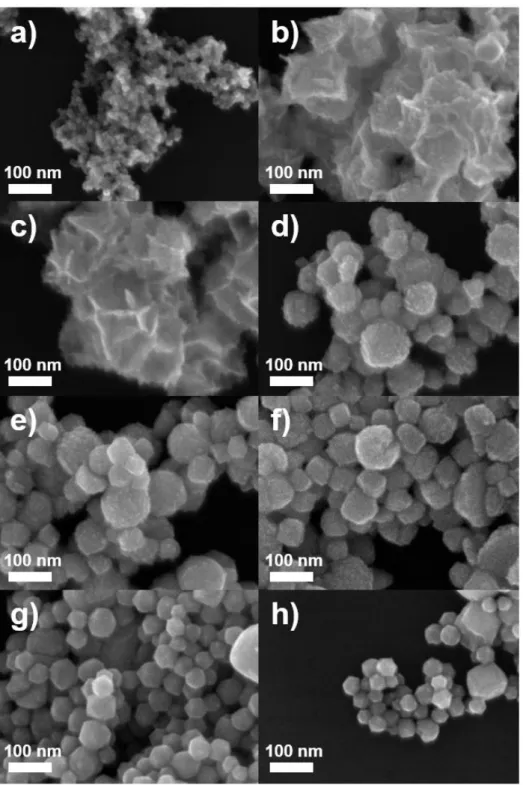
When a lower amount of CTAC (1.0 mL, 1.0 mM) than that used in standard synthesis nanosheets resulted in the formation of ultrathin Pd-Pt composite nanosheets (Figure 5b) with a very similar shape to standard ultrathin Pd-Pt composite nanosheets ( Figure 5c), while the use of a higher amount of CTAC (1.0 mL and 200 mM) resulted in 3D nanostructures with irregular shapes (Figure 5d–h). Ultrathin composite Pd−Pt nanoplates (Fig. 6a–d) were obtained using a lower concentration of AA and 0.1 mL, 50 mM) compared to that under standard conditions (Fig. 6e), while increasing the amount of AA to 1, 0 and 2.0 ml produced nanostructures with random sizes and shapes (Figure 6g-h). At high CTAC concentration, CTAC can interfere with the adsorption of I-ions on the surface of Pd-Pt nanostructures.
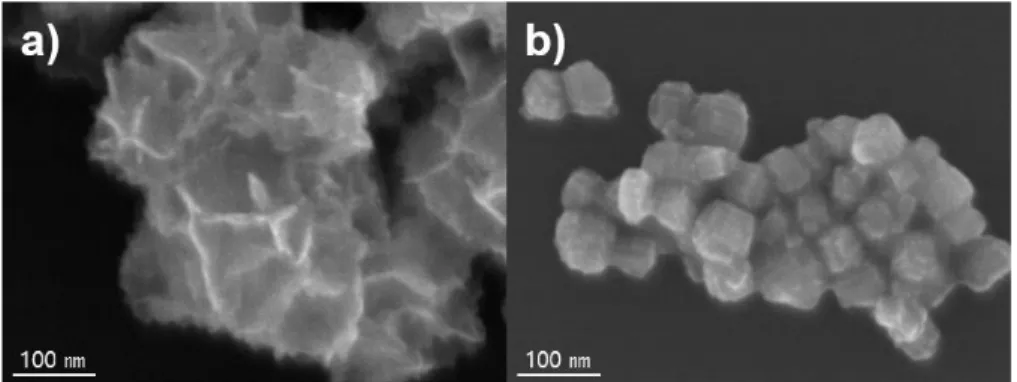
These results of control experiments collectively confirm that the synthesis of Pd−Pt composite nanosheets with ultrathin thickness can be realized by controlling the growth behavior of Pd-Pt through modulation of the KI, CTAC and AA concentration. We present standard methods for synthesizing Pd-Pt ultrathin composite nanosheets (UANs) using 0.2 mL of 50 mM KI, 1 mL of 5 mM CTAC, and 0.2 mL of 50 mM AA solution. In addition, we synthesized Pd-Pt irregularly shaped nanocrystals (IS NCs) to compare the effect of the ultrathin nanosheet part of hierarchical shape.
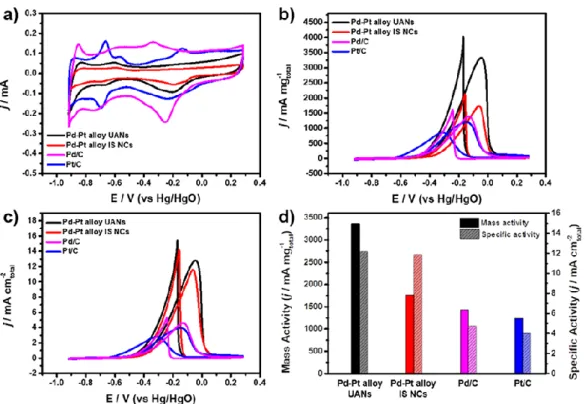
Assembled ultrathin Pd-Pt nanosheets (UANs) were used as electrocatalysts for the ethanol oxidation reaction (EOR) to investigate their morphological benefit. The EOR activity of assembled ultrathin Pd-Pt nanosheets (UAN) was evaluated in comparison with irregularly shaped Pd-Pt nanocrystals (IS NCs), commercial Pd/C and Pt/C catalysts. Although the ECSA value of assembled ultrathin Pd-Pt nanosheets (UAN) was smaller than both commercial catalysts, it was significantly larger than that of irregularly shaped Pd-Pt nanocrystals (IS NCs) due to ultrathin nanosheet part.
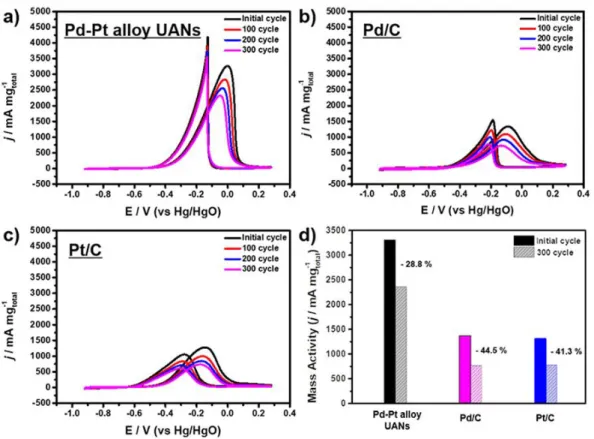
In this regard, the mass activities of Pd-Pt ultra-thin assembled nanosheets (UANs) were higher than commercial Pd/C and Pt/C. In addition, Pd-Pt ultrathin assembled nanosheets (UANs) showed higher mass activity than Pd-Pt irregularly shaped nanocrystals (IS NCs). The electrochemical stability of Pt-Pd ultrathin assembled nanosheets (UANs) was estimated by repeating CV and their properties were compared with different catalysts (Figure 9).
The Pd-Pt ultrathin composite nanoplates (UANs) show the best stability among the different catalysts of EOR activity.
Conclusion
34;Oleylamine-Mediated Synthesis of Pd Nanoparticles for Catalytic Formic Acid Oxidation." Journal of the American Chemical Society 131, No. 34;Controlled Formation of Concave Tetrahedral/Trigonal Bipyramidal Palladium Nanocrystals." Journal of the American Chemical Society 131, No. 34; Shape-controlled Pd nanocrystal-polyaniline heteronanostructures with modulated polyaniline thickness for efficient electrochemical ethanol oxidation.” Journal of Materials Chemistry A 7, no.
34; Peptide-assisted 2-D assembly toward free-floating ultrathin platinum nanoplates as effective electrocatalysts." Nano Energy.