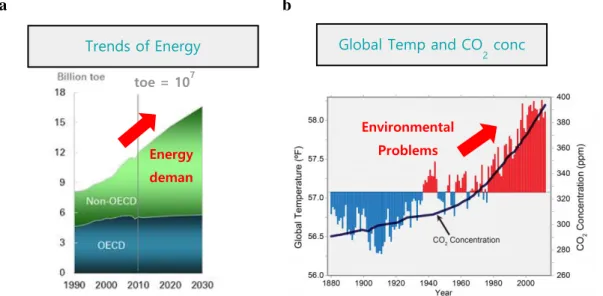
Configuration of transition metals in perovskites: A new strategy to enhance catalytic activity for the oxygen evolution reaction and stability in water. As hydrogen has attracted attention as a next-generation energy source due to its high energy density and environmentally friendly power generation process, research into hydrogen production technologies such as feedstock reforming has been actively conducted. fossil fuels, biohydrogen and water electrolysis, etc. Among them, a water splitting system which is one type of water electrolysis is considered a desirable hydrogen generating device because it enables continued hydrogen production without pollution.
However, for the commercialization of a water splitting system, the development of a cost-effective and efficient electrocatalyst for the oxygen evolution reaction (OER) and long-term sustainability are important. Recently, many researches have been conducted to use perovskite oxides as electrocatalysts replacing conventional noble-based catalysts due to the large catalytic activities, structural stability, low cost, and diverse doping capabilities of perovskite oxides. Moreover, there have been many researches that are about to improve the electrochemical catalytic activities of perovskite oxides, such as controlling the morphology of perovskite oxides, doping elements at A/B sites, synthesizing composite materials, and so on.
In this thesis, a composite catalyst composed of perovskite oxide and electron donor was designed by means of physicochemical synthesis method, which improves the performance of water splitting system by improving the catalytic activity for OER by controlling the electronic configuration of transition metal in perovskite oxides. Physicochemically controlled electron configuration of transition metals in perovskites: A new strategy to enhance the catalytic activity for oxygen evolution reaction and durability in water splitting system. Black spheres are substrate materials, white balls mean zirconium spheres that physically transform the substrate.
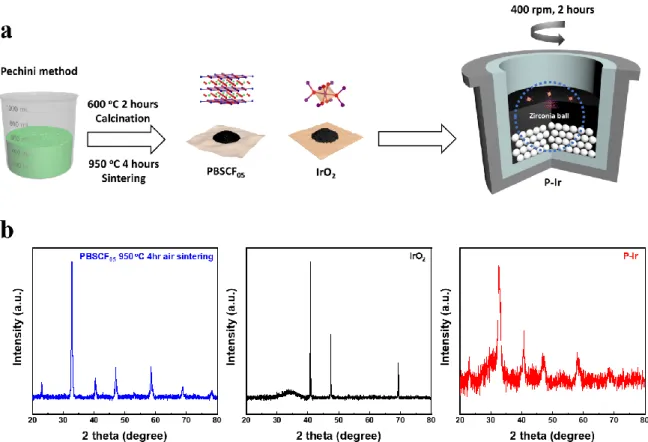
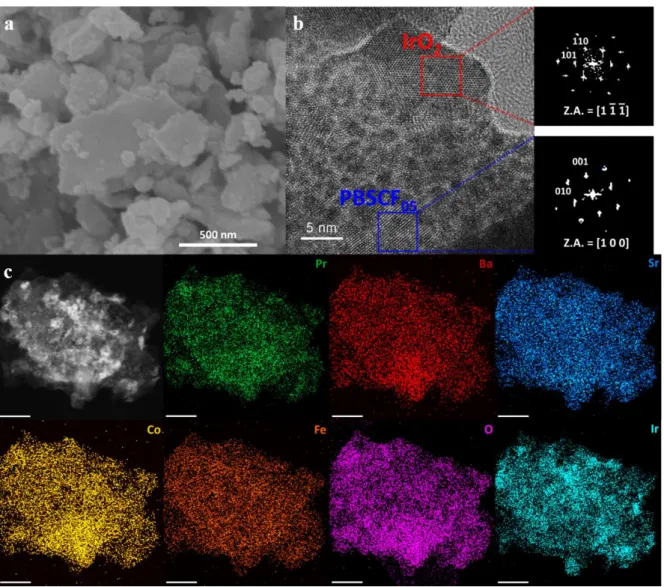
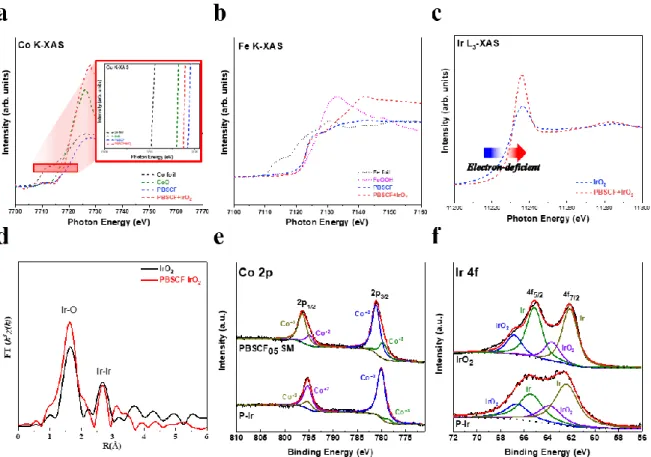
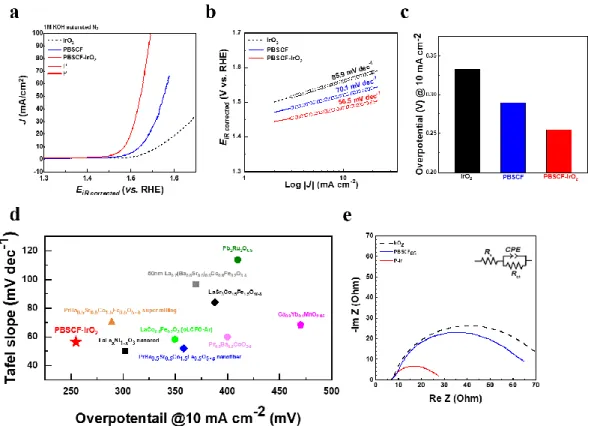
A schematic image of synthetic process of PBSCF-IrO2 and (b) XRD pattern of PBSCF, IrO2 and PBSCF-IrO2. c) The elemental mapping for PBSCF-IrO2; scale bars of 50 pm. a) Co K-XAS spectrum and (b) Fe K-XAS spectrum of PBSCF and PBSCF-IrO2. d) The corresponding Fourier transform of Ir L-edge EXAFS oscillations k2(k). e) Co 2p XPS spectrum of PBSCF and PBSCF-IrO2. d) OER activity comparison graph showing the Table Slope with overpotential@ 10 mA cm-2 for PBSCF-IrO2 and reported state-of-art oxide-based electrocatalysts.
Introduction
- Motivation statement
- Hydrogen generation technology: Water electrolysis
- Water splitting system
- Mechanism of water splitting system
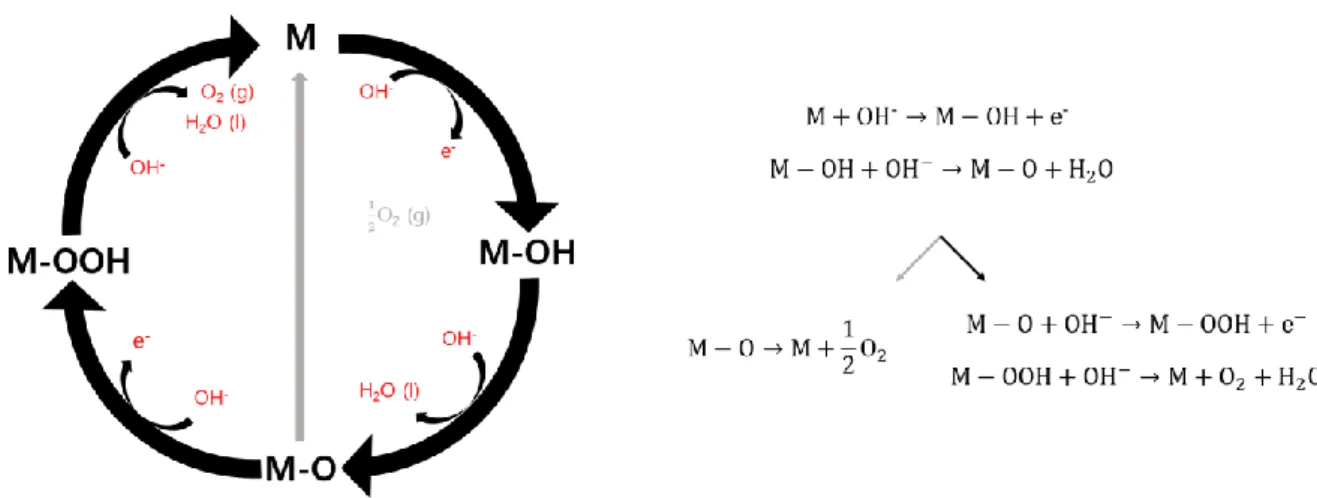
- Oxygen evolution reaction
- Selection of electrocatalyst for oxygen evolution reaction
- Reference
In particular, water electrolysis is considered a desirable hydrogen production device for practical use because it enables continuous hydrogen production without the risk of resource depletion. According to Table 1.1, water electrolysis is classified into 3 types of electrolytes: alkaline water separation system (in short, water separation system), polymer electrolyte membrane electrolysis (PEM electrolysis) and high temperature electrolysis (THE) [5]. Among them, the water splitting system is easy to scale up and economical compared to other electrolysis.
In an alkaline water splitting system, two main reactions take place: hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Furthermore, it is easy to scale up to large production system due to the simple structure and mechanism of the water splitting system. However, the practical application of the system is hindered due to the slow kinetics of OER, relatively.
Thus, the bottleneck phenomenon of OER occurs and affects the overall water splitting process, slowing down the rate of hydrogen production[7]. Therefore, the improvement of OER activity should be carried out for efficient water splitting process for commercialization. In general, noble metal-based catalysts, such as Ir and Ru-based catalysts, still remain the reference catalysts for the OER[9].
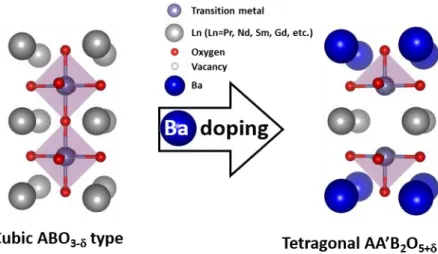
Although these materials exhibited great catalytic activity for OER, their extremely high cost and poor stability during OER have limited the application of noble metal-based catalysts on a commercial scale. Therefore, it is necessary to develop electrocatalysts with low noble metal content combined with other materials that exhibit high catalytic activity and relatively low cost combined with sufficient stability for OER, rather than the use of a noble metal-based catalyst alone. In particular, ABO3-type perovskite oxides (A: rare earth or alkaline earth elements, B: transition cation) have gained enormous attention as alternative candidates for noble metal-based electrocatalysts due to various doping abilities, good structural stability, and relatively low cost [6,10].
In addition, it has been reported that changing the electronic configuration of the transition metal in perovskite oxides affects the catalytic activities [11]. In-situ local phase-changed MoSe2 in La 0.5 Sr 0.5 CoO3-δ heterostructure and stable total water electrolysis in 1000 hours. Recent advances in mechanism understanding and design of electrocatalysts for alkaline water splitting.
Single-atom and cluster-based nanomaterials for hydrogen evolution, oxygen evolution reactions and complete water splitting. Physicochemically controlled electron configuration of transition metals in perovskites: a new strategy to stimulate catalytic activity.

Physico-chemically Controlled Electron Configuration of Transition
Result and discussion
- Crystalline Structure and Morphology
- Electrocatalytic activities
Conclusion
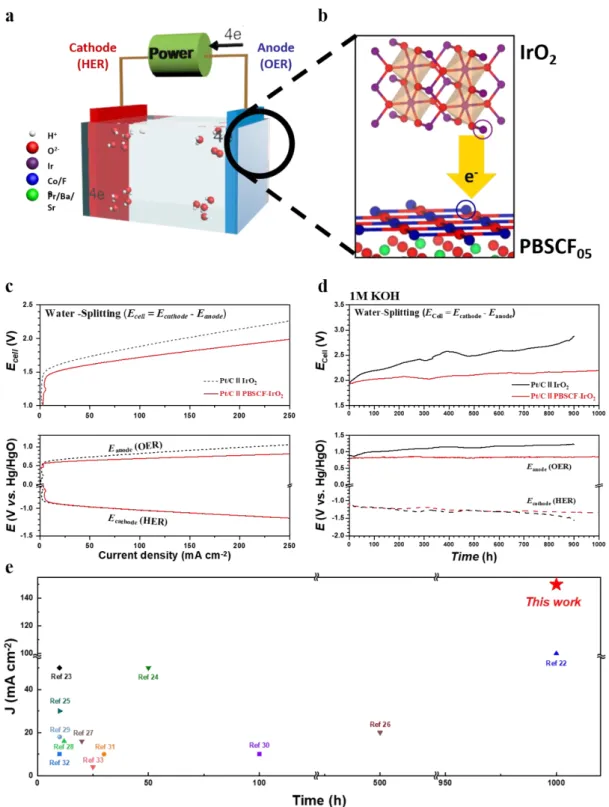
In summary, we reported the PBSCF-IrO2 composite catalyst which was synthesized via physicochemical super-milling treatment with PBSCF and IrO2 in this work. In the half-cell test, the PBSCF-IrO2 complex catalyst exhibited excellent electrochemical catalytic activity with an onset potential of 1.43 V, Tafel slope of 53.5 mV dec-1, and overpotential of 0.25 V at a current density of 10 mA cm -2, compared to IrO2. In addition, the PBSCF-IrO2 composite catalyst exhibited superior long-term stability around 1000 h without degradation in the water splitting system.
In addition to a simple and easy-to-large-scale production synthesis method, the outstanding electrocatalytic activity for OER and the durability of PBSCF-IrO2 in water splitting system suggest the possibility of efficient production and practical application of next-generation hydrogen energy source.