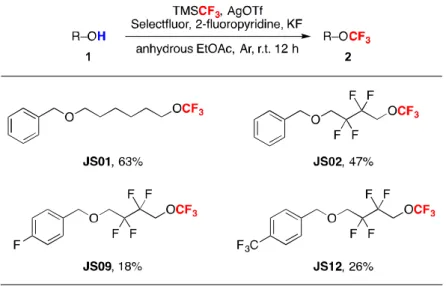
Due to the high electronegativity of the fluorine atom, the introduction of fluorine into the organic molecule affects the properties. According to the Qing group, reported in 2015, they have demonstrated an efficient and practical method to achieve O-trifluoromethylation of various alcohols. In addition, we also tested those ethereal compounds as Li-metal battery additives with the help of Prof.
Due to the inherent limitations of Li-ion batteries, the development of post-energy storage systems is highly studied. Li-metal is considered the next generation of Li-based batteries because it has the highest theoretical capacity and low electrochemical potential. Fluoroethylene carbonate (FEC) is one of the representative electrolyte additives in Li-ion batteries, but unlike the Li-ion system, carbonate-based additives are not the best choice.
We also found articles on the use of quercetin as an electrolyte additive in Li-ion batteries. Introduction of trifluoroethyl group with hypervalent iodonium salts is considered to be a promising method due to the electrophilicity, non-toxicity and easy handling. Due to the functional groups in the structure, they have been studied as attractive structural scaffolds and many methods have been constructed.
Here, we report the metal-free site-selective O-trifluoroethylation of various quinazolinone derivatives with the hypervalent iodonium salt under mild conditions.
Introduction
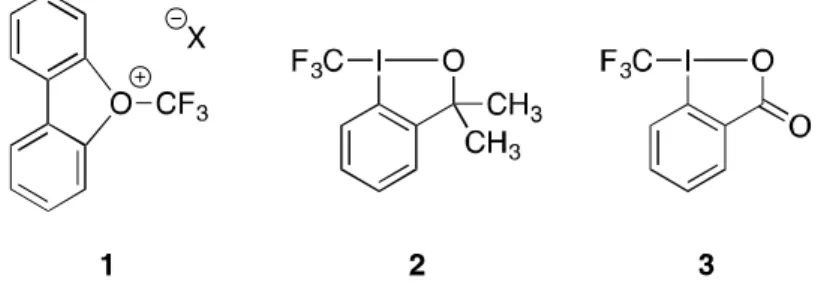
Transition metal catalyzed reactions also have a serious problem with the formation of carbonyl difluoride and metal fluoride. Nevertheless, the formation of O-CF3 bond from alcohol is difficult due to the difficulty of orbital overlap with oxygen and carbon, and the strong electronic repulsion between oxygen and fluorine, resulting in the formation of alkoxy halide. Recently, the Togni group developed a novel synthetic strategy for electrophilic trifluoromethylation based on the hypervalent iodine(III) derivatives (2, 3,3-dimethyl-1-(trifluoromethyl)-1,2-benziodoxole and 3, 1- trifluoromethyl-1,2-benziodoxol-3-(1H)-one, figure 1.1).
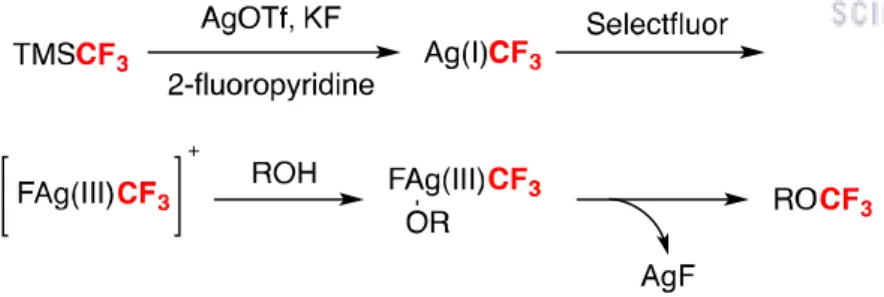
In 2009, Togni and zinc-mediated direct O-trifluoromethylation of alcohols to ethers with the Togni II reagent. However, the conversions of the starting materials were poor due to the excess amount of alcohols, and the use of co-solvent showed even less yield.[3-4,6] Therefore, the development of an efficient and practical route for the direct trifluoromethylation of alcohols to ethers is strongly required. In 2015, Qings group reported a silver-mediated oxidative trifluoromethylation of alcohols to trifluoromethyl ethers with the Ruppert-Prakash reagent (TMSCF3).[5] They demonstrated the trifluoromethylation of various primary, secondary, and tertiary alcohols in moderate to excellent yields at room temperature and inert conditions.
They proposed a mechanism by which a trifluoromethyl silver complex is formed in situ, not an alkoxy silver complex, and after an oxidation step with Selectfluor from Ag(I) to Ag(III), reductive elimination occurs and the desired product is obtained (Scheme 1.1). Based on Qing's report, we report here the formation of trifluoromethyl alkyl ethers and the use of the compounds as anode lithium metal electrolyte additives.
Results and Discussions
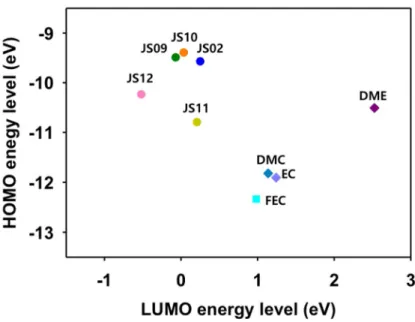
Subsequently, trimethylsilyl alkyl ether compounds that are known to be efficient additives for fluorinated electrolytes were synthesized (Table 1.2). Since the role of the additives is to form a stable SEI layer and maintain the electrolyte, lower LUMO energy levels of the additives than the electrolyte are certainly needed. All candidates showed lower LUMO value than 1,2-dimethoxyethane (DME), so Li/Cu cell life cycle tests were performed (Figure 1.2).
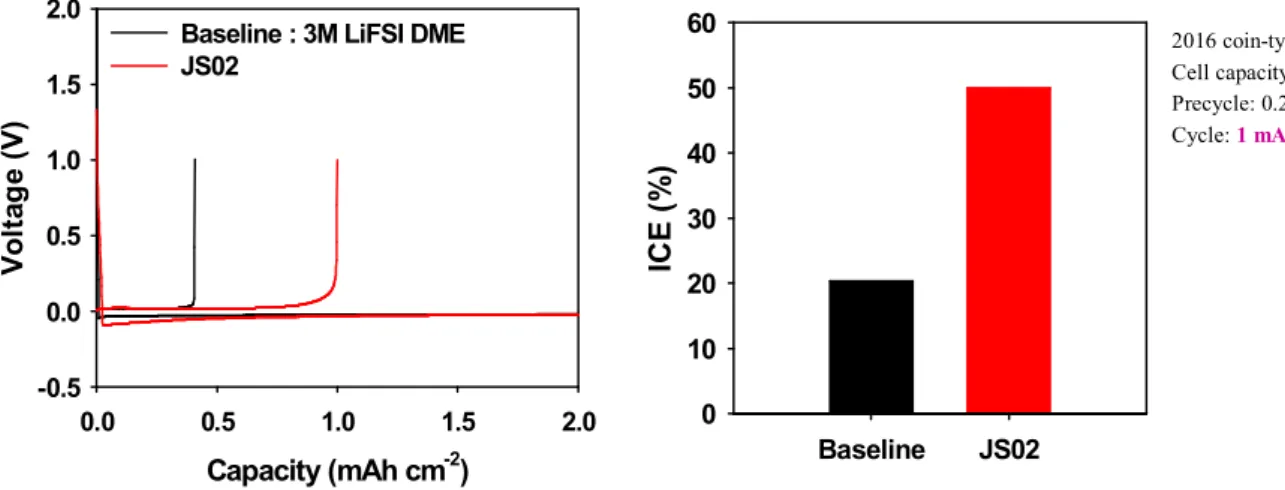
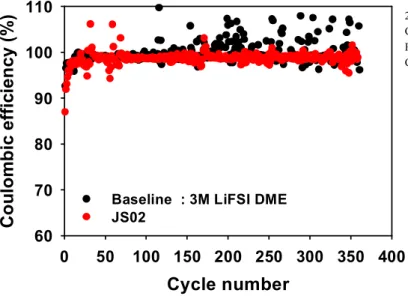
Before the battery cycle, a pre-cycle was performed to form a uniform SEI (Figure 1.3) and the initial coulombic efficiency of the added JS02 electrolyte showed a marked improvement compared to the baseline. Furthermore, the coulombic efficiency of the added JS02 cell is consistently maintained up to 350 cycles, while the baseline value started to fluctuate from 200 cycles (Figure 1.4).
Conclusion
Synthesis and application of baicalein derivatives for Li–metal
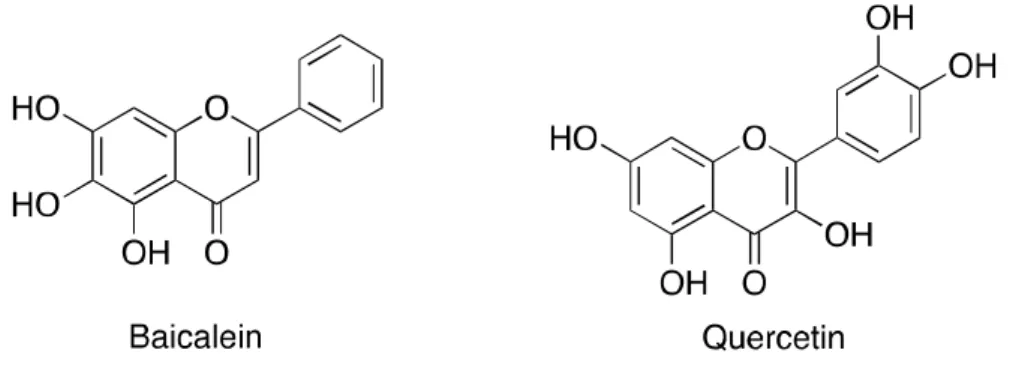
Unlike Li-ion batteries, ethers are more suitable for Li-metal anode systems than organic carbonates. They show higher CE and affect dendrite suppression, however, due to the low anodic decomposition (<4 V vs Li+/Li) and high flammability, which is neglected in most commercial batteries.[1]Therefore, it is desirable to have a new types additives for Li-metal battery systems. Surprisingly, there are few reports of quercetin, one of natural flavonoids showing antioxidant properties, as an electrolyte additive in Li-ion batteries.
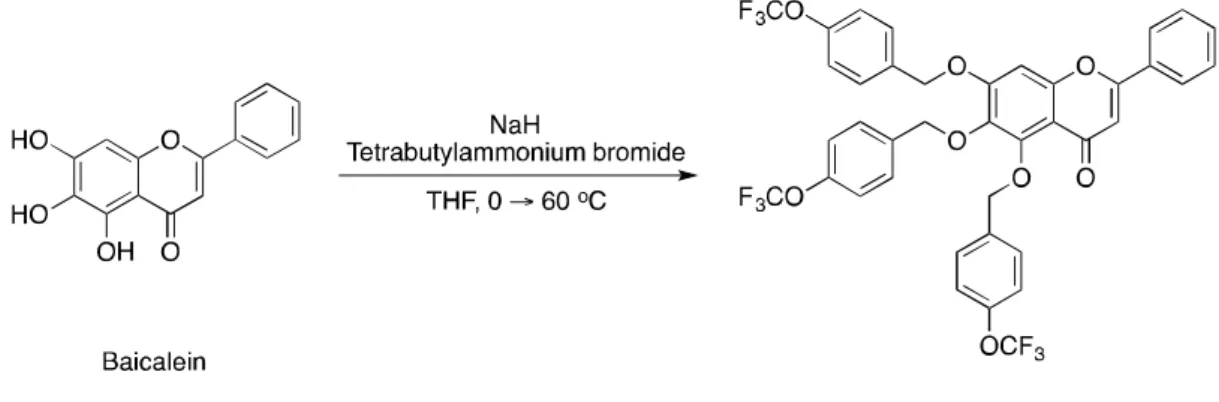
Lee et al. reported the significant improvement of the safety and lifetime of Li-ion batteries by using quercetin as an electrolyte additive.[13]. Inspired by the reports of using quercetin, one of the flavonoid derivatives, as Li-ion battery additive, we used baicalein derivatives containing ether functional groups as Li-metal battery additives. To compare the performance of additive candidates, fluorinated additives were also synthesized and applied to Li-metal batteries.
To synthesize substituted I-4, it is necessary to prepare substituted benzaldehydes, and they were linked to I-2 through aldol condensation. Therefore, it is necessary to optimize the condition to achieve higher yields in an efficient manner in the near future. Then all additive candidates were tested for the use of Li-metal battery additives.
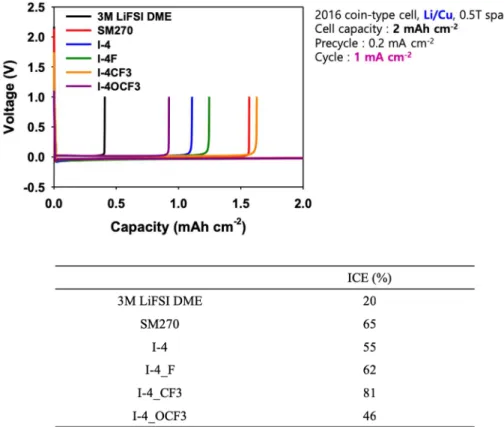
As shown in Figure 2.3, all candidates appear to meet the requirements, so battery life cycle tests were performed. Therefore, it is necessary to find the optimal condition for combining series I-4 and fluoroethylene carbonate (FEC) to raise ICE. After the previous cycle, battery life cycle tests were performed to see the effect of the additives on the electrolyte (Figure 2.5).
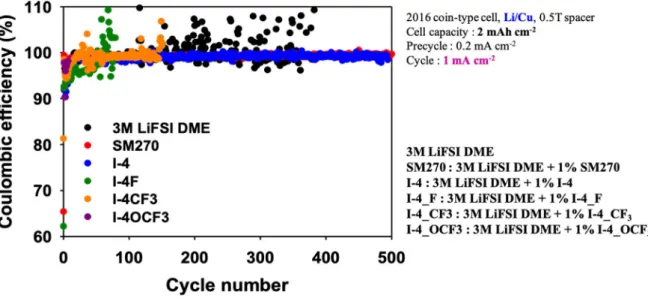
See Figure 2.5, a combination of FEC and SM-270 or I-4 candidates appears to have influential possibilities as promising Li-metal battery additives. Finally, it is necessary to continue further analysis to reveal the reasons for discrepancy between ICE and CE. In summary, we have synthesized a few derivatives of baicalein molecules, I-4 series and SM-270 for use as Li-metal battery additives.
Since the initial Coulombic efficiency of all candidates did not show more than 90%, it is recommended to combine with another organic additive material, such as fluoroethylene carbonate (FEC). Compared to the additive-free standard condition that showed stable Coulombic efficiency up to 200 cycles, only SM-270 and I-4 cells showed a remarkable stability of Coulombic efficiency and cycle life.
Late-stage metal-free O–trifluoroethylation of quinazolinone
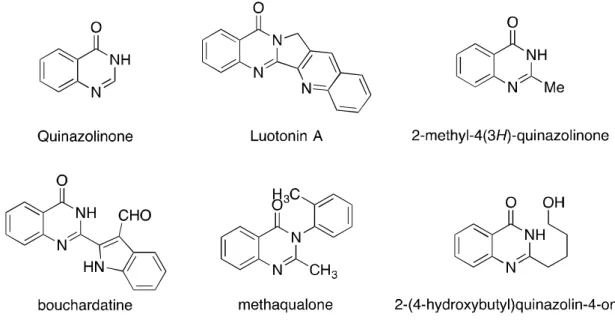
Quinazolinone derivatives are widely present in natural products, for example luotonin A from Peganum nigellastrum and 2-methyl-4(3H)-quinazolinone from Bacillus cereus (Figure 3.2). In addition, the derivatives show biological and pharmacological activities such as anti-tumor, anti-HIV, anti-inflammatory and antibacterial.[9-13] Due to the properties of quinazolinone derivatives, they have been developed as an interesting structural fence and many methodologies have been developed. However, to the best of our knowledge, there is no example of constructing a metal-free O-trifluoroethylation with a trifluoroethyl group containing a hypervalent iodonium salt.
Most reactions were completed within 4 h, whereas a few substrates required an additional 2, K2CO3 and reaction time to fully consume the starting materials (Table 3.1 entries d, f, g, h, j). All substrates showed excellent yields and other halogen atom and methyl group substituted arenes showed good yields (Table 3.1, entries d-g). Substituents on the right side ring effect were also investigated (Table 3.1, h–o) and showed moderate to excellent yields.
In summary, we have demonstrated metal-free and site-selective O-trifluoroethylation of various quinazolinone derivatives using a mild-mode hypervalent iodonium salt. To see the generality of the reaction, O-trifluoroethylation of substituted arenes on the left or right side (a–g vs. h–o) and the position of the substituents on the arenes were performed and they did not affect the yield of the desired product. Therefore, we believe that this late O -trifluoroethylation of quinazolinones can be applied in medical field for diversification.