In this work, new conducting systems based on hydrogel polymer with tunable electrical and mechanical properties were developed. More specifically, the individual contributions of polyacrylamide (PAAm), chitosan (CS), phytic acid (PA) and polyaniline (PAni) to the enhancement of electrical sensitivity and stability of the hydrogel under strain were investigated. PAAm, and chitosan with superior mechanical properties and biocompatibility, are widely used hydrogel materials, however, their strainability and electrical sensitivity are limited.
Therefore, PA and PAni are two compounds that can increase the electrical sensitivity and mechanical stability of hydrogels. Therefore, the synthesis of conductive polymer-based hydrogel and its characterization, including mechanical properties and electrical sensitivity were studied in detail. Specimens were cut into rectangular pieces s for the tensile test, as well as for the electrical sensitivity test (a).
34 Figure 4.3.3. Graphical results of electrical sensitivity test with open .. chronoamperometric technique by stretching hydrogels for 1 minute.
Introduction
Aim of research
This research aims to produce a new CP-based hydrogel for wearable strain sensors through rational design of polyacrylamide, chitosan, phytic acid and polyaniline (PAni), a conducting polymer, to generate material with higher strain sensitivity and stability. Optimize the hydrogel synthesis by introducing PAni into the PAAm-chitosan-phytic acid matrix to achieve the required mechanical and electrical properties. Evaluate the mechanical and electrical properties of the hydrogel as well as its sensitivity to strain using various characterization techniques, including scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), and mechanical testing.
To test the performance of the hydrogel as a wearable tension sensor in real-world conditions and assess its potential applications in domains such as healthcare, sports and robotics. Due to their high electrical conductivity and ability to undergo reversible conductivity changes when subjected to mechanical stress or pressure, conductive polymers, such as PAni, are vital for making hydrogels for wearable voltage sensors. By integrating PAni into the hydrogel matrix, it is possible to generate a material that is both biocompatible and conductive, making it perfect for use in a variety of applications, especially in the medical field.
The incorporation of PAni into the hydrogel matrix can improve its effectiveness as a portable strain sensor by increasing the sensitivity of the hydrogel to strain.
Hypothesis and goals
Literature review
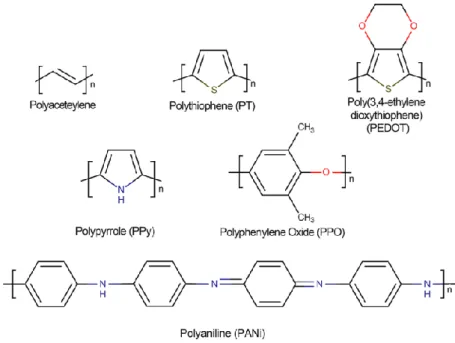
- Conductive polymers: polyaniline
- Polysaccharides: Acrylamide, Alginate, and Chitosan
- Phytic acid
- Conductive Polymer Hydrogels
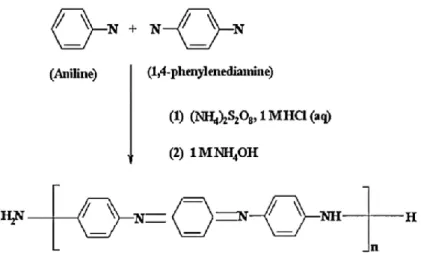
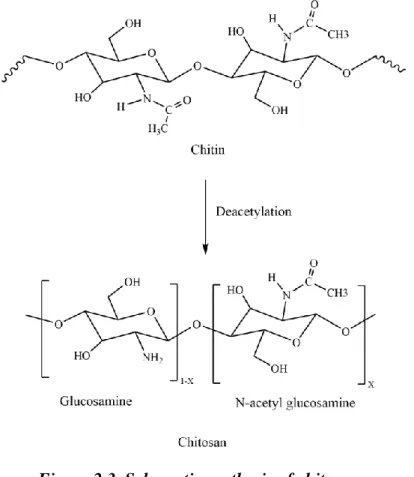
During the process of obtaining PAni, the polymer can be in three states according to its components: leucoemeraldine [(C6H4NH)n], emeraldine [([C6H4NH]2[C6H4N]2)n] and per-nigraniline [(C6H4N)n ], where Emeraldine is the most conductive. Polymers are generally categorized in various ways, but the basic categorization used in the pharmaceutical industry divides them into natural and synthetic polymers. According to Figure 2.3, the linear structure of chitosan, which is obtained from chitin by deacetylation, consists of amino and hydroxyl groups for reaction with other polymers in solution.
SA can function as a polyelectrolyte to provide the hydrogel with strong conductivity in addition to its good biocompatibility. The obtained hydrogel sensor was biocompatible and could be used to detect different human movements. The hydrogel produced by adding SA showed greater tensile stress than a hydrogel produced without adding SA and consequently it is a suitable choice to create wearable hydrogel sensors [23].
In addition, hydrogels produced from synthetic polymers have attracted much interest over the years because of their positive properties over those of hydrogels produced from natural polymers. Since the structure can be modified according to its effectiveness, hydrogels composed of synthetic polymers have improved mechanical properties, long life before degradation, and significant water absorption capacity [24]. Due to its strong antioxidant activity and ability to chelate Fe3+ ions, phytic acid (PA), which is extracted from oilseeds, legumes, grains, nuts and pollen by acidic solutions while heated and/or stirred and then purified, has positively demonstrated health and physiological benefits.
As a result, phytic acid has received more attention for its many documented benefits than for its traditional label as just an antinutrient. Polymeric networks, known as hydrogels, can hold and absorb large amounts of water because they contain hydrophilic groups that hydrate in an aqueous medium to create a hydrogel structure. Cross-linking is necessary to avoid the breakdown of the polymer chains before use, as the word "network" is implied.
Doping is a key technique in the modern semiconductor industry because it enables the electronic characteristics of semiconductors to be tuned. The polaronic or bipolaronic structure along the polymer chain, or the balance between polarons and bipolarons, is caused by the acidic dopant that protonates the imine groups in PAni.
Materials and Methods
- Materials
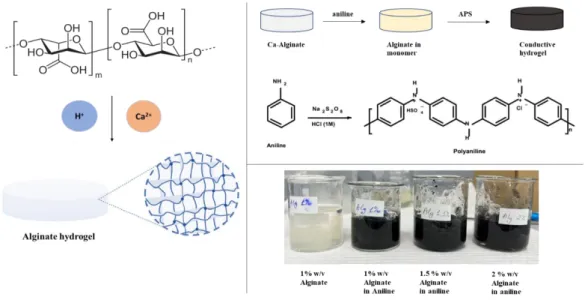
- Preparation of Alginate hydrogel with and without conductive polymer
- Preparation of Alg-PA hydrogel with and without conductive polymer
- Preparation of dual-crosslinked Alg-PA hydrogel with and without conductive
- Testing mechanical properties of hydrogels
- Fourier Transform Infrared (FTIR) analysis
- Scanning Electron Microscope (SEM)
- Swelling property test
- Conductivity test
- Antibacterial test
Consequently, the obtained hydrogels, having the black color, were washed with acetone to remove unwanted unreacted molecules. The process of preparing Alg-PA hydrogel is similar to the previous method, but the phytic acid played the role of crosslinker and gelator instead of calcium chloride. As mentioned previously, 3 different volumes of PA (160 μl, 200 μl and 250 μl) were added dropwise to 2% w/v 20 ml viscous solutions of alginate under magnetic stirring and left for 24 h for hydrogel maturation.
In the next step, the obtained hydrogels were soaked in aniline for one day, and in the APS oxidant solution for 24 hours the next day. Next, these gels were soaked in a CaCl2 solution, which should cross-link with unbound alginate molecules to produce a stronger hydrogel. The resulting viscous solution is cooled to 00 C to add 0.005 g of KPS initiator.
Synthesis of PAAm/PA-CS hydrogel with CP includes the whole process above, and after obtaining a strong and stretchable gel, it was soaked in 0.4 M Ani-HCl solution, which. A tensile test was performed (by TA.XTplus, Stable Micro Systems, SIMAS™) to check the tensile properties of hydrogels and measure strain. For these measurements, hydrogels were cut into rectangular shapes (30 mm x 10 mm x 4 mm) and stretched at a running speed of 1 mm per second. p.
The analysis of the chemical properties of the materials was done using the Nicolet iS10 FT-IR spectrometer. Lyovapor™ L-200, BUCHI was used for the preparation of the materials by freeze-drying technique for 24 h and the ZEISS Crossbeam 540 scanning electron microscope was used for the analysis of the surface morphology of the cross-sectional sections, which were coated with gold in 10 nm. The swelling ability of the hydrogels was tested and measured during the synthesis of the PAAm/PA-PAni hydrogel, where the materials were immersed in a 0.4 M Ani-HCl aqueous solution with a weak acidic medium.
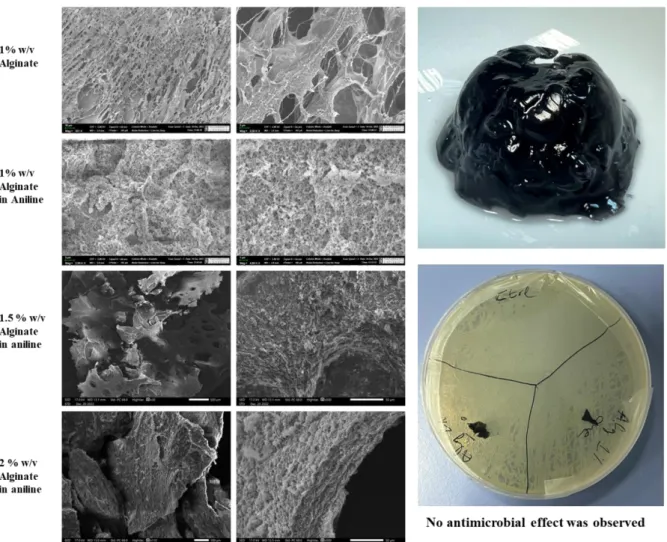
The antimicrobial properties of obtained hydrogels were tested on Gram-negative Escherichia coli (E. coli) and Gram-positive bacteria Staphylococcus aureus (S. aureus) and Bacillus subtilis (B. subtilis). To observe the inhibition of samples, the plates were incubated in an oven at 37oC for 24 hours until the total growth of bacteria.

Results and Discussion
- Alginate hydrogel sample preparation with CaCl 2 and Phytic acid
- PAAm/PA-CS hydrogel preparation with conductive polymer (PAni)
- Testing mechanical properties and electrical sensitivity of hydrogel
- Conductivity test
- Swelling properties
- FTIR analysis
- Morphology analysis by Scanning Electron Microscopy
- Antibacterial test
Schematic representation of the formation of hydrogen bonds with the addition of phytic acid to alginate solution. In Figure 4.1.5, it is shown that the addition of a PA crosslinker leads to the synthesis of weak hydrogel, which started to destroy when rinsed with DI water. It is explained that hydrogen bonds of phytic acid and electrostatic interactions between molecules possibly contribute to the adhesive property of hydrogels [28].
For the first step of the synthesis, different volume ratios between phytic acid and DI water were chosen, as shown in Figure 4.2.1C, where the total volume of solution for the hydrogel synthesis is 10 ml. In addition, no gel was formed in the PA:DI (2.5:7.5) ratio due to phytic acid deficiency, which can be clearly seen in Figure 4.2.2b. The effect of increasing phytic acid content can be determined by respectively increased strain levels of samples in graphs a-c.
The stress level of 75 kPa and 550% strain was shown by the hydrogel sample without phytic acid (0:10). The increase in phytic acid content may be the main reason for the decrease in strength, which can be seen in the graph for the ratio 7.5:2.5 (without PAni), where the strength rate decreased significantly to a number of ± 4 0.7 kPa and 6 ± 1.8 kPa of modulus. The result of the resistance measurement is demonstrated in Figure 4.4.1, where significant differences can be observed between the samples with and without PAni.
This phenomenon can be explained by the role of phytic acid as a dopant for aniline monomer, which exhibits electrical conductivity in addition to an ionic conductivity of PA. An increase in phytic acid content in hydrogels can reduce the swelling property due to higher density and low porosity. According to Figure 4.6, the peaks at 1563 and 1471 cm-1 in the FTIR spectrum of polyaniline (black line) correspond to the C=N and C=C stretching modes of the quinoid and benzenoid rings, respectively.
Figures 4.7.1b and 4.7.1c show the 3D porous spongy structure in hydrogels with a 5:5 ratio of phytic acid and water, without and with PAni. In addition, it can be appreciated that alginate and PAni are highly biocompatible polymers and that the very low antimicrobial activity comes from the small amount of phytic acid. Consequently, the microbial zone of inhibition can expand with the increase of phytic acid content in a hydrogel, which is clearly shown in Figure 4.8.2.
Excellent antimicrobial activity was clearly demonstrated in Figure 4.8.2, where hydrogels with a ratio of 5:5 and 7.5:2.5 showed the mean radius of the zone of inhibition 1.55 mm and 1.67 mm against E.
Conclusion
Hodgson et al., "Reactive Supramolecular Assemblies of Mucopolysaccharide, Polypyrrole, and Protein as Controllable Biocomposites for a New Generation of. Wan et al., "3D Conducting Polymer Platforms for Electrical Control of Protein Conformation and Cellular Functions," J. Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications,” Acta Pharm.
![Figure 2.4. Chemical structure of Phytic acid . Reprinted with permission from [25]](https://thumb-ap.123doks.com/thumbv2/azdokorg/10332557.0/17.892.393.554.101.262/figure-chemical-structure-phytic-acid-reprinted-permission.webp)