Matlamat kajian ini adalah untuk menjerap ion Cr, Cd dan Mn dalam larutan akueus menggunakan komposit nanomagnet aluminium oksida (Al) menggunakan dua kaedah berbeza iaitu pencampuran fizikal (PM) dan pemendakan kimia (CP). Komposit Al nanomagnet, iaitu alumina berdop nikel (NiAl), alumina berdop kobalt (CoAl), dan alumina terdop besi (FeAl), digunakan dalam proses penjerapan untuk membandingkan prestasinya terhadap ion Cr, Cd dan Mn. Kadar penjerapan ion Cr, Cd dan Mn adalah dalam susunan berikut: FeAl > CoAl > NiAl > Al.
Kinetika dijelaskan oleh model orde pertama semu, sementara studi termodinamika menunjukkan bahwa adsorpsi adalah eksotermik untuk Cr, sedangkan Cd dan Mn adalah endotermik. Penelitian ini bertujuan untuk menghilangkan ion Cr, Cd dan Mn dari larutan berair menggunakan komposit nanomagnetik alumina (Al) menggunakan dua metode yang berbeda yaitu pencampuran fisik (PM) dan presipitasi kimia (CP). Adsorptivitas adsorben terhadap ion Cr, Cd dan Mn adalah sebagai berikut: FeAl > Karbon > NiAl > Al.
The best model to explain the adsorption of Cr, Cd and Mn ions was the Langmuir model.
INTRODUCTION INTRODUCTION
Background of the study
Adsorbents such as activated carbon, silica, zeolite, clay and graphene have been synthesized and applied for the treatment of pollutants (Burakov et al., 2018; Garshasbi et al., 2017). According to a recent study, nanomaterial composites have a large surface area with a higher capacity for adsorption due to their porosity and small diameter (Gusain et al., 2020). All that attracts potential as a good adsorbent for wastewater treatment is one of the effective nanomaterial adsorbents due to its high porosity and large surface area (Ali et al., 2019).
Despite the focus on nanomaterials, the separation of the used adsorbents after treatment was difficult due to their smaller nanosize. To solve these problems, nanomagnetic metal oxides were introduced due to their high capacitance and selectivity (Liu et al., 2016). Metal oxides that exhibit magnetism properties can be reused in the adsorption process, enhancing industrial property value by saving money for acquiring more raw products.
For example, magnetic boehmite sol-gel composites improved the removal of heavy metals (Shapovalova et al., 2018). Therefore, the aim of this study is to synthesize Fe, Co, and Ni loaded on Al for the adsorption of Cr, Cd, and Mn ions. In this study, the physicochemical properties and adsorptivity of Fe, Co, and Ni metals deposited on Al were studied by PM and CP methods.
Based on these results, the structure of the compounds and the adsorption mechanism were proposed.
Problem statement
- General objective
- Specific objectives
Then various parameters, including contact time, pH, initial concentration, adsorbent dosage and temperature, were investigated. To synthesize the nanomagnetic Al adsorbents by loading Fe (FeAl), Co (CoAl) and Ni (NiAl) metals using two different methods of PM and CP. To characterize the nanomagnetic Al (FeAl, CoAl, NiAl) adsorbents using XRD, FTIR, SEM, BET, XPS and ESR.
To investigate the optimal conditions for the adsorption of Cr, Cd, Mn ions using the synthesized adsorbents, including contact time, pH, initial concentration, adsorbent dosage and temperature.
Research significance
Introduction
Heavy metals
- Chromium (Cr)
- Cadmium (Cd)
- Manganese (Mn)
For example, improper control of the discharge of widely used palladium (Pd) in industry leads to the death of aquatic life (Salleh et al., 2015). However, plants do not require it because they do not produce lipids or sugars (Das et al., 2021). Cd is one of the main pollutants that is highly toxic in drinking water that comes mainly from industrial wastewater and incineration (Ali et al., 2019).
Cd is also used in the production of batteries, anticorrosive agents and the production of pigments (Al Hamouz et al., 2017). Mn is a natural element that can be found widely in the environment and an essential micronutrient for the human body and organisms (Tran et al., 2018). When exposed to oxygen, the red-brown color and water-insoluble nature of Mn become apparent (Nadia et al., 2020).
Mn contamination in water sources has become a serious problem as global steel production has increased (Nadia et al., 2020). Mn accumulation in specific brain areas is linked to neurotoxicity and degenerative brain diseases (Yahya et al., 2020). In addition, Mn intake during pregnancy can be harmful, leading to issues such as DNA damage, respiratory problems and even manganese-induced parkinsonism (Tran et al., 2018).
Excess Mn also stains cookware, clothing, toiletries, and contaminated food supplies, discolored clothes, and gave drinking water a metallic flavor (Tran et al., 2018). Thus, the flow of water is interrupted, which reduces water quality and increases distribution costs (Nadia et al., 2020). Thus, the maximum concentration of Mn in drinking water allowed by the World Health Organization was 0.05 mg L-1 (Tran et al., 2018).

Technology of heavy metals remediation
Reversible ion exchange between solid and liquid phases can occur, releasing H+ from functional groups to facilitate metal complexation with the free functional group (Abdullah et al., 2019). Due to the simplicity of treatment principles, ease of operation and resilience in large water volumes and high concentration wastewater, electrolysis is often used for the fundamental removal of heavy metals from industrial wastewater (Abdullah et al., 2019). The application of a coagulant or chemical in wastewater causes a chemical reaction, resulting in coagulation (Shrestha et al., 2021).
Small flocs formed by slow mixing of water can grow and settle in the solution (Shrestha et al., 2021). Although it is a simple and non-metal selective heavy metal separation process, it generates a large amount of sludge and poses a separation problem by transferring the hazardous chemicals to the solid phase (Abdullah et al., 2019). This procedure can be classified as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis based on pore size (RO) (Nazaripour et al., 2021).
The membrane separation process is mainly governed by three fundamental principles: adsorption, sieving and electrostatic phenomena (Nazaripour et al., 2021). Polymeric membranes are gaining popularity due to their attractive properties such as flexibility, excellent mechanical integrity and ease of production (Shrestha et al., 2021). In a sense, adsorption suggests that the solid (adsorbent) binds molecules of ions or substances through physical attraction, ion exchange and chemical bonding (Liu et al., 2021).
According to Liu et al. 2021), adsorption of heavy metals (adsorbate) occurs on the surface of adsorbents using ion exchange or chemisorption. The adsorption is also simple in design, flexible, the ability to reduce heavy metal concentration to the lowest level and easy operation (Shapovalova et al., 2018). Other conventional heavy metal removal techniques incur high capital costs, which were prohibitive for small scale industries (Ni et al., 2019).

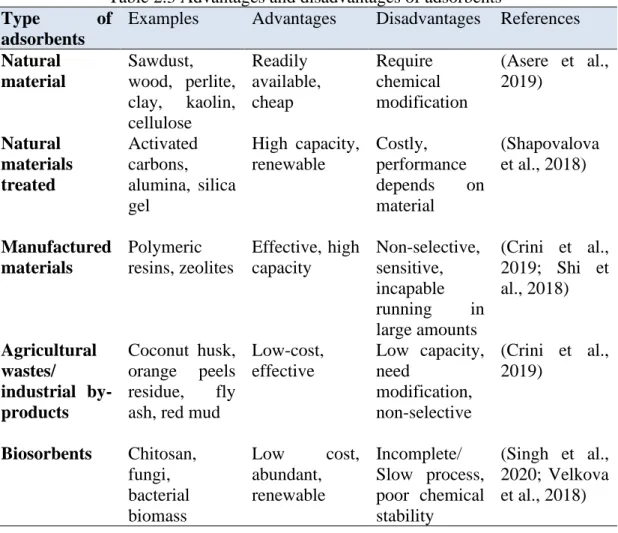
Adsorbents
However, the use of activated carbon is not feasible in wastewater treatment due to the high cost of commercial activated carbon charcoal and numerous process losses related to material regeneration (Shapovalova et al., 2018). Carbon nanotubes (CNTs) are another adsorbent that proves to be efficient in the removal of heavy metals such as Pb (II) and Cu (II) (Nyairo et al., 2018). Due to its cation-rich and high surface area, zeolite is one of the potential adsorbents in the petrochemical industry, refining catalysis and gas purification (Shi et al., 2018).
However, adsorbents are sensitive, non-selective and cannot flow in large quantities (Crini et al., 2019). Agricultural solid waste and industrial by-products such as date pits, fly ash and red mud are also promising materials due to their low cost and efficiency. As evidence, the appearance of various agricultural wastes such as wheat, husks, leaves, corn dust and fruit wastes such as banana peels (Crini et al., 2019).
Coconut husk, a common agricultural waste in Malaysia, useful to solve waste management problems because it is abundant, cheap and has a high surface area due to being rich in fiber (Malik et al. , 2017). As a low-cost adsorbent, orange peel contains lignin, cellulose components, hydroxyl and carbonyl groups in the structure. Moreover, the reuse of waste fly ash solves the issue of air pollutants as it is considered as an irritant (Marinina et al., 2021).
They act as an excellent adsorbent to collect elemental and toxic wastes from the environment, including industrial wastes, heavy metals, fertilizers, pesticides and air pollutants (Singh et al., 2020). As a result, the possibilities of implementing continuous biosorbent processes for metal removal are reduced, and the practical application of biosorption in industrial situations was limited (Velkova et al., 2018). On the other hand, inorganic transition materials have attraction as useful adsorbents, namely AB2O3 general structure of mixed metal oxide, for example nickel aluminate (NiAl2O4) (Salleh et al., 2015).
Nanomaterials and alumina (Al)
Gamma Al (γ - Al2O3) is of interest because it has a large surface area and is very stable over the temperature range of most catalytic processes. Catalyst supporters can benefit from the inherent acid-base properties, attractive mechanical characteristics and variable surface physicochemical properties of Al (Tabesh et al., 2018). Al is an adsorbent that can also support other materials composite material with the ability to form active sites (Salleh et al., 2017).
The advantage of the large surface area makes Al a popular carrier for metal ions. It has been reported that modified Al can remove various dyes, heavy metals, inorganic effluents and many others in the industry. Even less work has been reported on the function of Al as a ceramic carrier in composite materials.
A comparison of Al-modified biomasses of agricultural residues for Ni and Cd removal was performed using three biomasses of agricultural residues (maize cob, orange peel and oil palm bagasse) modified with Al nanoparticles. Increase in capacity adsorption confirmed the suitability of these Al-modified biomasses for the removal of heavy metal ions (Herrera-barros et al., 2021).

Al composites and magnetism