I certify that this project report entitled “EFFECT OF CALCINATION DURATION ON THE PHYSICAL AND CHEMICAL PROPERTIES OF MOLYBDENUM-DOPED VANADIUM PHOSPHORUS OXIDE CATALYST” prepared by OW CHUN ONN, JONATHAN has met the required standard for submission in partial fulfillment of the requirements for the award of Bachelor of Engineering (Hons.) Chemical Engineering at Universiti Tunku Abdul Rahman. I would like to thank everyone who contributed to the successful completion of this project. Once again I would like to thank everyone who directly or indirectly contributed to the completion of this project.
This project aims to investigate the effect of different calcination duration on molybdenum dopant on the physical and chemical properties of vanadium phosphorus oxide (VPO) catalyst.
Catalysis and Catalysts
Types of Catalysis
Homogeneous Catalysis
Heterogeneous Catalysis
In a catalyst, for example, the catalyst is a solid, usually a noble metal such as platinum or rhodium. However, the substances on which the catalyst acts are gases, such as nitrogen(II) oxide and other gaseous combustion products. Another industrial example would be the production of benzene from the dehydrogenation of cyclohexane using platinum-on-alumina as a catalyst [Science Clarified, 2010].
Energy Profile of Reaction with Catalysts
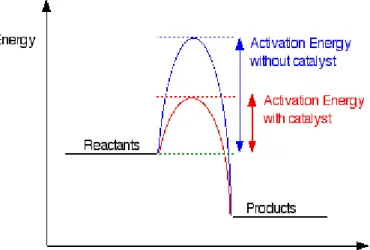
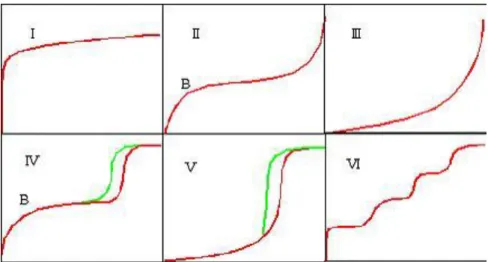
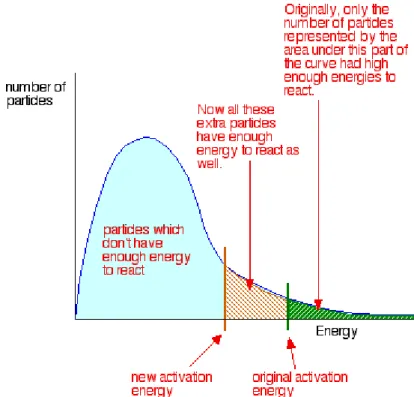
A catalyst provides an alternative pathway for the reaction, and this alternative pathway has lower activation energy as shown in Figure 1.1 [Clark, 2002]. For gases, the Maxwell-Boltzmann Distribution graph shows the distribution particle energies in each particle. From the Maxwell-Boltzmann Distribution graph, it is noted that only a handful of particles have energy high enough to react.
By adding a catalyst, which provides an alternative route with lower activation energy, more particles are able to react and the Maxwell-Boltzmann Distribution graph is shifted to the left [Clark, 2002].
Essential Properties of Good Catalysts
Importance and Uses of Catalysts
Problem Statements
Objectives of Research
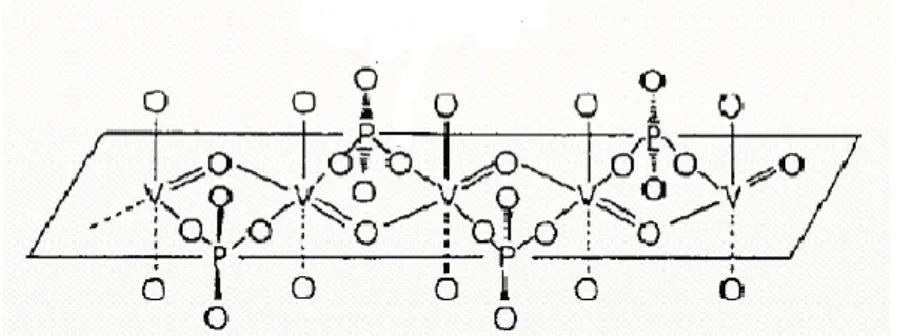
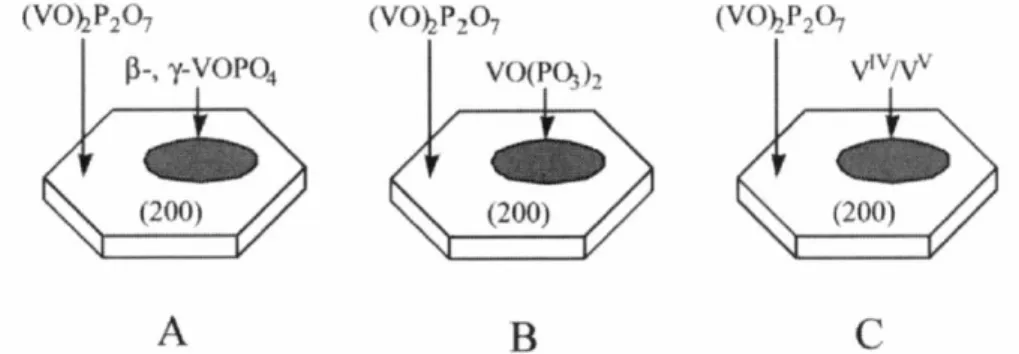
Active and selective surface sites for the oxidation of n-butane to maleic anhydride were associated with the presence of vanadyl dimers on the (2 0 0) surface planes of (VO) 2P2O7. 1987) believed that the active sites are not associated with the interfaces between these crystalline phases. They suggested that the active phase for the selective oxidation of n-butane consists of a well-crystallized mixture (VO) 2P2O7. 1997) suggested that the active sites for the oxidation of n-butane to maleic anhydride involve a V4+/V5+ couple well distributed on the surface of a variety of VPO phases.
This precursor was converted to an amorphous VO(PO3)2 catalyst, which is much less active but as selective as the (VO)2P2O7 catalysts (Figure 2.1B). 1988) attributed the activity of the VPO catalysts in n-butane oxidation to vanadyl pyrophosphate, whereas the selectivity toward maleic anhydride was associated with the presence of a very limited and controlled amount of V5+ sites (Figure 2.1C).
Preparation of Vanadium Phosphorus Oxide Catalyst
- Aqueous Medium
- Hemihydrate Precursor Route
- Dihydrate Precursor Route
- Organic Medium
- Sesquihydrate Precursor Route
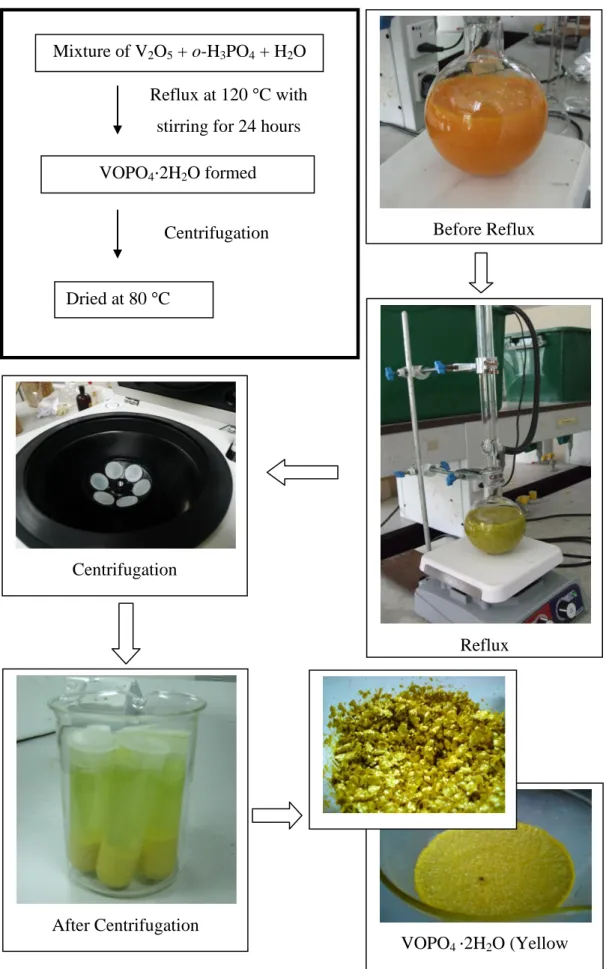
When vanadium phosphorus oxide (VPO) catalyst is prepared via aqueous medium, the final catalyst tends to have a low surface area where the catalyst activity is directly proportional to surface area. Dihydrate precursor, VOPO4∙2H2O, method was first proposed by Horowitz et al. 1988) and further described by Johnson et al. 1997) reported that very active catalysts can be prepared using a two-step method based on VOPO4·2H2O, which is known as the VPD route. According to Bartley et al. 2001), dihydrate precursor (VOPO4∙2H2O), as a starting point for catalyst preparation, is ready to achieve effective control of the hemihydrate precursor (VOHPO4∙0.5H2O) morphology.
Therefore, the VPD route can yield the (VO)2P2O7 phase with the highest platelet surface morphology, which exposes a preferred (1 0 0) active plane for maleic anhydride formation [Hutchings et al., 1997]. One of them is by providing a method that produces a high-surface-area form of VOHPO4·0.5H2O that can be topotactically transformed into a final high-surface-area catalyst comprising mainly (VO)2P2O7. In addition, the precursor also provides a new route to the synthesis of VO(H2PO4)2, which has been found to provide ultraselective catalysts for the synthesis of maleic anhydride [Hutchings et al., 1997].
Catalysts prepared in an organic medium will show a higher specific reactivity, as they not only have a higher surface area, but also a greater density of active sites [Cavani and Trifiro, 1994]. By using organic medium in the production of this catalyst, it is possible to obtain high values of surface area of precursors, which can be increased by calcinations at 380oC, and characterized by a lower oxidizability in air at high temperature. After calcinations, it had shown high specific activity in selective oxidation of n-butane to maleic anhydride [Taufiq-Yap et al., 2004].
The synthesis of sesquihydrate precursor is divided into a two-step procedure using VOPO4 2H2O as an intermediate before obtaining the precursor. After cooling to room temperature, the resulting precipitates are centrifuged from the solvent, washed sparingly with acetone and oven dried to obtain the sesquihydrate precursor [Taufiq-Yap et al., 2004].
Parameters of Vanadium Phosphorus Oxide Catalyst
- Parameter: Calcination Conditions
- Parameter: Support System
- Parameter: Dopant
- Parameter: P/V Atomic Ratio
Support system in VPO catalyst is a method to increase the yield and selectivity of the catalyst for converting n-butane to maleic anhydride. Therefore, many studies have been devoted to determining the dispersion, wetting and interaction of active species with support materials [van Santen et al., 1999]. Dopants, which are also known as promoters, are a popular choice in VPO catalyst to improve the surface area and morphology of the catalyst to increase reactivity and selectivity.
The method of adding promoters was investigated by Batis et al. 1996) where promoter additions are based on thermal addition, Lewis acid promoted addition and others. On the other hand, the electronegativity of the promoter has been highlighted by Takita et al. 2000) where the activity of the catalyst is based on the property of the charge and the electron acceptor. Mechanistically, the strong Lewis acid sites favoring hydride abstraction, which is the rate-determining step, may be a V4+δ+ site derived from defects in the vanadyl pyrophosphate [2 0 0] planar structure according to Centi et al.
Nevertheless, most authors have researched and concluded that the addition of dopants in the VPO catalysts increased the surface area of the catalysts and induced the formation of V5+ phases which were shown in XRD and LRS spectra which promoted the catalytic performance [Pierini and Lombardo, 2005]. During the preparation of VPO catalyst, it was found that the P/V atomic ratio plays an important role in the reaction over VPO catalysts [Ballarini et al., 2006]. However, there is a lack of discussion about the structure of these catalysts and details of the conversion in different conditions.
Tousi and Mahdavi (2010) found that the synthesis of the organic medium is a favorable method for the preparation of the catalyst, and both the activity and the selectivity are directly dependent on the P/V ratio of the catalyst structure. This effect is related to the increase of the surface area and crystallinity of the VPO sample and the increase of the acidity of Bronsted (P–OH) and Lewis acid sites (V4+).
Maleic Anhydride
Uses of Maleic Anhydride
Maleic anhydride (MA) is widely used in the production of polyester resin, as MA is an important intermediate for the processing of unsaturated polyester. Polyesters are used in many fiber-reinforced plastics; materials with a wide and growing range of applications in the marine, automotive and construction industries. It is also used as a co-monomer for unsaturated polyester resins, an ingredient in binders used in the production of plywood, a corrosion inhibitor and a preservative in oils and fats.
In addition to polyesters, MA is also important in the production of alkyl resins used in paints and coatings. In addition, it is used as an additive to lubricating oils, which are used in crankcase oils for gasoline and diesel engines as dispersants and corrosion inhibitors [PrimaryInfo.com, 2009]. MA is also used in water treatment chemicals, detergents, certain agricultural chemicals such as insecticides and fungicides, pharmaceuticals and copolymers.
Oxidation of n-butane to Maleic Anhydride
- Reaction Details
Benzene is a known carcinogen and one of the most heavily regulated chemicals by the government. Flammability limits for C4 hydrocarbons are also lower than those for benzene, which is an additional process safety advantage. For all these reasons, the fixed-bed process with n-butane has been the only MA route used commercially since 1985 in the United States [Lee, 2009].
The commercially predominant butane oxidation process uses a fixed bed, but processes using fluidized beds and transport beds are also being developed. In the fixed-bed process, a low concentration of butane is passed over the catalyst in tubular reactors similar to the benzene process. The reactor is operated at temperatures between C and the pressure is maintained at 0.3 - 0.4 MPa to force the exit gases downstream for scrubbing and cleaning.
Unlike the benzene process, only a small amount of the MA can be condensed from the reactor effluent due to the increased formation of water. The MA is then distilled from the high boiling solvent in a fractional distillation column [Lee, 2009].
Materials and Gases used
Methodology
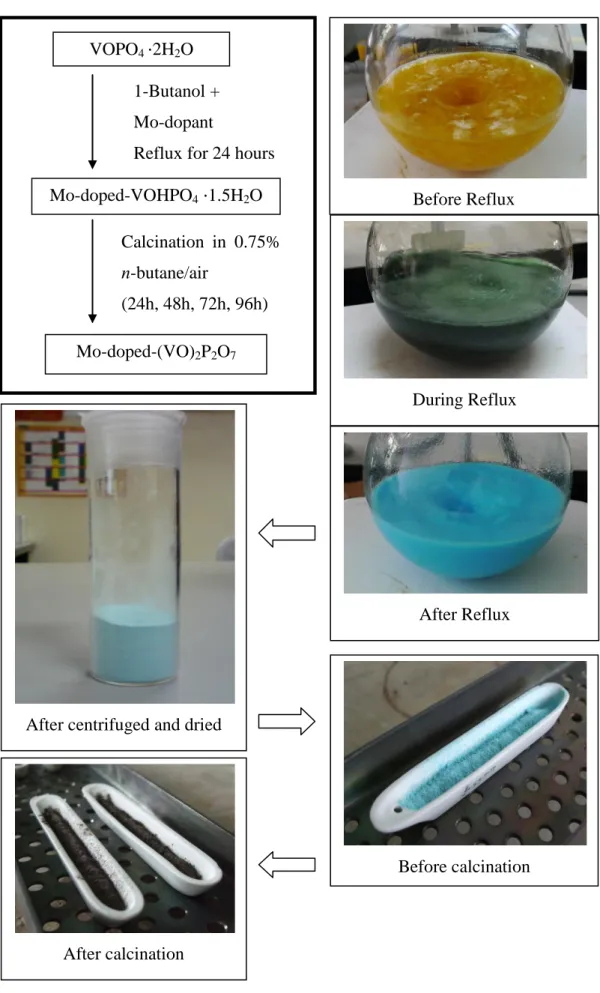
Preparation of the Mo-doped Vanadium Phosphorus Oxide Catalysts
Characterisation Techniques and Instrumentations
- X-Ray Diffraction (XRD) Analysis
- BET Single Point Surface Area Measurement
- Redox Titration
- Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES)
- Scanning Electron Microscopy-Energy Dispersive X-Ray Spectroscopy (SEM-EDAX)
It is one of the most powerful and effective techniques for qualitative and quantitative analysis of crystalline compounds. It is designed to perform both physisorption and chemisorption, enabling the determination of total specific surface area, porosity and specific surface area. When a material is exposed to a gas, an attractive force acts between the exposed surface of the solid and the gas molecules.
The surface area of a solid includes both the external and internal pore surfaces [CE-Instrument UK, 2001]. The filling of the first layer enables the measurement of the surface of the material, because the amount of gas absorbed when the monolayer is saturated is proportional to the entire surface of the sample. The endpoint was reached when the purple color of the solution disappeared and became colorless.
Then another 25 ml fresh of the original solution was treated with 0.01 N ammonium iron(II) sulfate solution. Secondary electrons are emitted from the atoms occupying the upper surface and produce an easily interpretable image of the surface. Due to the small diameter of the primary electron beam, a high-resolution image can be obtained [SCIS, 2010].
The contrast in the image produced is determined by the atomic number of the elements in the sample. Interaction of the primary beam with atoms in the sample causes shell transitions, which result in the emission of an X-ray beam.

X-Ray Diffraction (XRD)
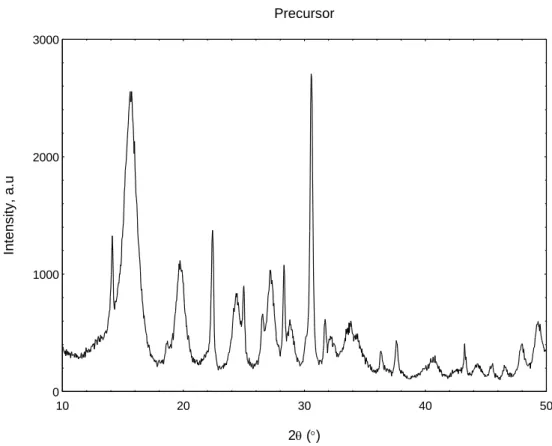
The decreasing trend of the FWHM of the (0 2 0) planes indicates that the thickness of the particles in the (1 0 0) direction is decreasing. Longer calcination duration results in VPO catalysts with larger crystallite sizes in the (0 2 0) direction, but the crystallite sizes in the (2 0 4) direction are almost the same as the difference is small.
BET Surface Area Measurements and chemical analyses
Scanning Electron Microscopy (SEM)
Conclusions
Recommendations