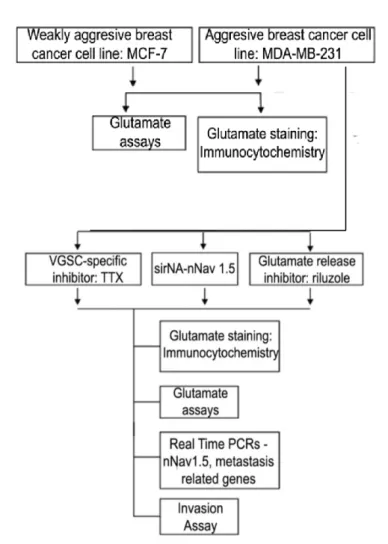
Peningkatan ekspresi dan aktiviti saluran natrium berpagar voltan (VGSC), terutamanya varian neonatal, nNav1.5, dalam sel kanser payudara manusia secara amnya dikaitkan dengan keupayaan untuk metastasize. Ekspresi mRNA nNav1.5 dikesan dalam ketiga-tiga jenis sel, dengan ekspresi tertinggi juga dalam sel MDA-MB231 (p <0.01). Kesimpulannya, tahap ekspresi nNav1.5 adalah berkaitan dengan kandungan glutamat dan potensi metastasis sel kanser payudara.
Yang paling penting, penelitian ini adalah yang pertama menunjukkan fungsi kontrol nNav1.5 terhadap kadar glutamat dan potensi metastatik berikutnya dalam sel kanker payudara. Dengan demikian, tujuan keseluruhan dari penelitian ini adalah untuk menyelidiki kontrol pengaturan nNav1.5 pada konsentrasi glutamat dalam sel kanker payudara dan potensi metastatiknya. Karena MDA-MB-231 ditemukan memiliki konsentrasi glutamat tertinggi secara signifikan bersama dengan pengaturan ekspresi mRNA nNav1.5, sel menjadi sasaran penghambatan nNav1.5 menggunakan cara ;.
In conclusion, nNav1.5 expression correlates with glutamate concentration and metastatic potential of breast cancer cells.
Background of the study
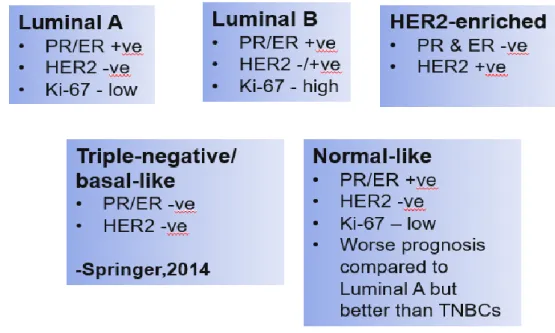
Nav1.2 SCN2A CNS, PNS Cervical, mesothelioma, ovary, prostate Nav1.3 SCN3A CNS, PNS Ovarian, prostate, small cell lung cancer Nav1.4 SCN4A Skeletal muscle Cervical, ovary, prostate. Breast, cervical, lymphoma, mesothelioma, non-small cell lung cancer, ovary, prostate Nav1.8 SCN10A sensory neurons Prostate. But later, when specific antibodies against nNav1.5, NESOpAb, were developed, the role of nNav1.5 in breast cancer was further defined (A.-M.
Furthermore, using all the agents mentioned, not only the downstream mechanisms regulated by nNav1.5 were revealed, but also the upstream mechanisms regulating nNav1.5 were elucidated (S. P. Fraser et al. 2014). Therefore, the utilization of TTX in terms of upstream regulation of nNav1.5 has been found to involve through the mechanism involving the transcription start sites - VGSCs contain the upstream promoter within a distinct cytosine-guanine island (CpG ) for co-expressed luciferase (Diss et al. 2008) e.g. However, this study was designed to investigate the downstream regulation of nNav1.5 in promoting breast cancer invasion.
Previously, several mechanisms were discovered such as pH regulation via sodium-hydrogen exchanger 1 (NHE1) – increased NHE activity lowers pH at the pericellular space which then induces proteolysis and ultimately promotes invasiveness (Brisson et al. 2011). Furthermore, decreased pH and the shift of H+ efflux were also able to activate cysteine cathepsin expression and promote epithelial-to-mesenchymal transition (EMT), respectively, all of which led to potentiation of metastasis and invasiveness of breast cancer cells (Luo et al. . 2020). As a result of the mentioned research, this study hypothesized that increased expression of nNav1.5 in breast cancer plays a role in the regulation of glutamate concentration and thus promotes invasion into cancer cells.
Instead of the hypothesis, it came to light that the increased concentration of glutamate was also associated with cell invasion, apart from a decreased pH or an increase in the activity of proteolytic enzymes to promote invasion (Sontheimer 2008; Yi et al. 2019). This ability is particularly true in neuronal cancers, where increased glutamate has induced excitotoxicity (as during brain injury) that promotes cell death/damage to surrounding cells to allow tumor spread (Noch and Khalili 2009; Ye and Sontheimer 1999). Increased expression and activity of VGSCs during excitotoxicity in brain injury is already well known, so we expect the study to show a toxic effect.
General objective
Breast cancer
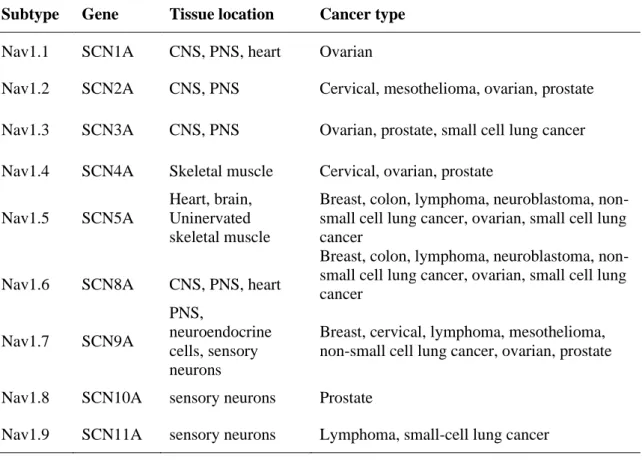
Conversely, low-income developing countries tend to have higher mortality even when they have a lower incidence (Bray et al. 2018). Among Association of Southeast Asian (ASEAN) countries, breast cancer is the leading cause of cancer mortality, accounting for 18% of all other cancers (American Cancer Society, 2015). These scenarios have created a disturbing situation as the incidence of breast cancer will continue to grow in the Asia-Pacific countries, including Malaysia (Youlden et al. 2014).
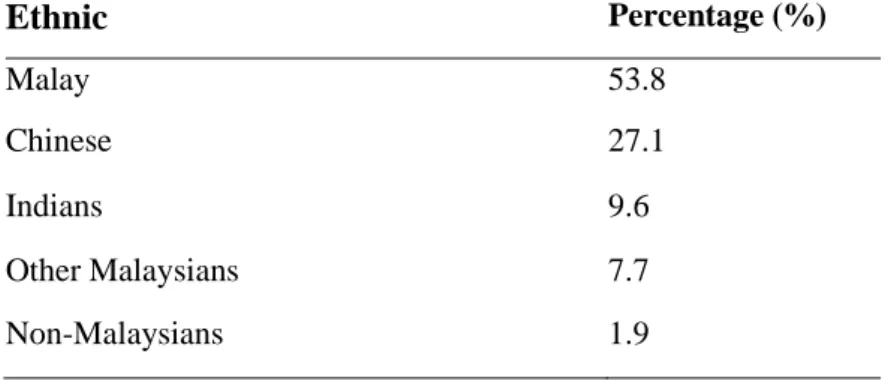
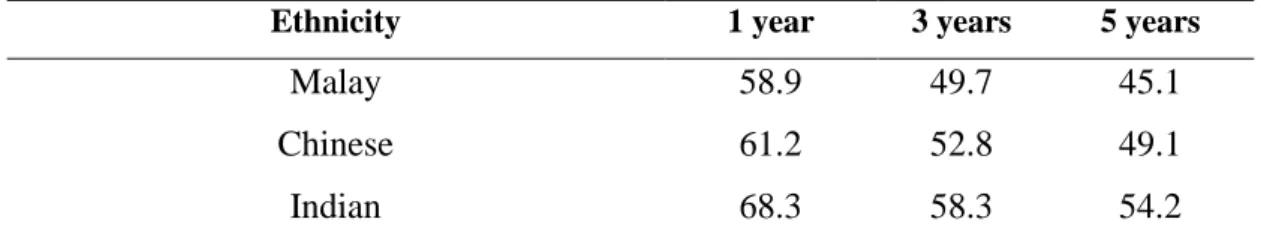
According to the latest data from the Malaysian National Cancer Registry, breast cancer is the leading cancer among women, followed by colorectal, cervical and ovarian cancer (Azizah et al. 2016). But it is worst when Malaysia is ranked among the worst breast cancer survival countries in the region (Abdullah et al. 2013) (Tables 2.1 and 2.2). Note: Breast cancer survival rates in developed countries such as the US, UK and Japan are.
Classification of breast cancer
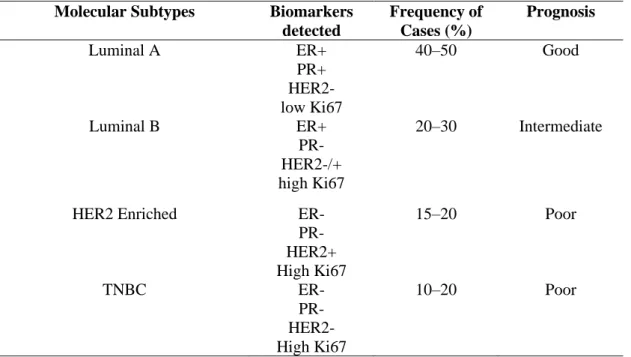
This classification is based on gene expression studies, where four distinct molecular characteristics or subtypes are used to define breast cancer subtypes (Vuong et al. 2014) (Table 2.3), Molecular targets specifically found in cancers such as hormone receptors, estrogen receptors (ER) and progesterone receptor (PR ) and Human Epidermal Growth Receptor 2 (HER2), the absence of which would be termed triple negative breast cancer (TNBC) (Schiff et al. 2003). A more distinct and consistent classification is achieved with molecular classification, which results in the better prognosis prediction and type of therapy to be administered based on presented biomarkers (Kanwar et al. 2020). Among those from the molecular classification, great interest has been placed on the triple negative breast cancer (TNBC) in general due to the poor prognosis associated with the subtype.
Worse, it increases the number of deaths, which is highly disproportionate to the incidence (De Laurentiis et al. 2010). The lack of common molecular targets has rendered conventional targeted therapies ineffective, leading physicians to rely on systemic therapy using cytotoxic agents (Lee and Djamgoz 2018). The aforementioned poor prognosis also stems from the tumor's characteristics, that it has a more antagonistic phenotype and a high propensity for metastatic development (Yao et al. 2017).
There is also considerable heterogeneity in TNBCs, slowing progress for a definitive targeted therapy (Caswell-Jin et al. 2020; Yao et al. 2017), differing in vital biological pathways and prognosis, of which 6 subtypes were derived; the basal-like 1 and 2 (BL1 and BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL) and luminal androgen receptor (LAR) (Lehmann et al. 2011).
Treatment of breast cancer
Meanwhile, adjuvant drug therapies are administered after surgery to kill any remaining cancer cells with the goal of reducing the chances of recurrence. This can be administered externally using machines to deliver radiation from outside the body or internally after surgery temporarily via a radiation delivery device near the tumor site (Ben et al. 2020). While progress has been made in the last decade, it remains an unfavorable method of therapy (Crown et al.
Although TNBCs are 'invincible' to most targeted therapies in metastatic cases, their high mitotic rate makes them somewhat sensitive to this type of treatment and more effective in an adjuvant or neoadjuvant setting (Hassan et al. 2010). Targeted therapy, as the name suggests, acts on molecular targets specifically found in cancers, such as hormone receptors, estrogen receptor (ER) and progesterone receptor (PR) and the Human Epidermal Growth Receptor 2 (HER2) (Schiff et al. 2003). In particular, HER2 upregulation has been shown to play an important role in the development and progression of certain aggressive types of breast cancer and gained prominence in 30% of breast cancer cases, significant enough to be designated as an important biomarker (Mitri et al.
Another monoclonal antibody, pertuzumab, was also approved by the FDA in June 2012 (Table 2.5) and can be combined with trastuzumab (Table 2.5) and also prevents dimerization in HER3 and HER2 (Baldo 2016). The next generation of treatments includes immunotherapy, which is the newest form of targeted therapy that deserves its own classification (Couzin-Frankel 2013). Other antibodies approved by the US FDA for breast cancer immunotherapy are listed in Table 2.5.
Importantly, immunotherapy is suitable for the most difficult-to-treat subtype of breast cancer, TNBC, where the absence of three key receptors reduces the chances of targeted therapy, along with a high incidence of malignancy and metastasis. Due to the high specificity of target and immunotherapy, they are the preferred choice of therapies where induction of cell death or inhibition of growth and proliferation occurs without affecting normal cells, thus reducing toxicity and side effects in patients. Immunotherapy is suitable for TNBC, as the absence of three key receptors reduces the chances of targeted therapy, together with a high incidence of malignancy and metastases.
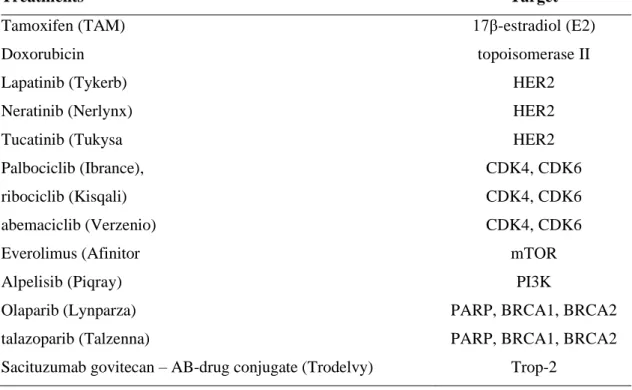
Hallmarks of cancer: Understanding cancer progression in search of tumour markers for cancer future management tumour markers for cancer future management
Cancer hallmark: Invasion and metastasis of breast cancer
Metastasis is a common cause of cancer death where effective treatment is not available that overcomes a number of barriers (Wei et al. 2013). Metastasis is defined as the spread of cancer cells from primary tumor sites to distant organs and tissues, which contributes to over 90% of mortality in cancer patients (Weigelt et al. 2005). Metastasis does not occur randomly, but rather by accumulating enough mutations to allow the metastatic cascade (Scully et al. 2012) begins with the local invasion around the primary tumor, detachment of the cells continues until it.
This acquisition of new traits occurs through unique transformation, from an epithelial to mesenchymal (EMT) and back (MET) cancer when it reaches a favorable secondary site (Scimeca et al. 2020). Therefore, in EMT, tumor cells lose their epithelial characteristics, cell-cell adhesion, epithelial polarity, with the acquisition of mesenchymal phenotype and migratory capacity (Guarino et al. 2007). Mutations in E-cadherin leading to its functional loss were discovered in lobular carcinoma of the breast (Keller et al. 1999).
Kotb et al. 2011) showed that expression of N-cadherin instead of E-cadherin resulted in the formation of fibrosis and cysts in mammary glands and eventually resulted in malignant mammary tumor in mice. Furthermore, as reported (Guarino et al. 2007), down-regulation of E-cadherin and up-regulation of N-cadherin were frequently observed in cancer cells of most epithelial cancers during stromal invasion. The development of distant metastases in various organs has become the leading cause of death from breast cancer (DeSantis et al. 2019; Lacroix 2006).
In three years after the first detection of the primary tumor, it was reported that about 10-15% of breast cancer patients will develop distant metastases (Weigelt et al. 2005). It has been reported that ER-positive breast cancer mainly invades the bone and, to a lesser extent, is detected in the brain and lung (Soni et al., 2015). The series of events explained above such as trait accumulations, EMT-MET-EMT transformations, detachment and adhesion are all part of the sequence of the metastatic cascade (Scully et al. 2012).
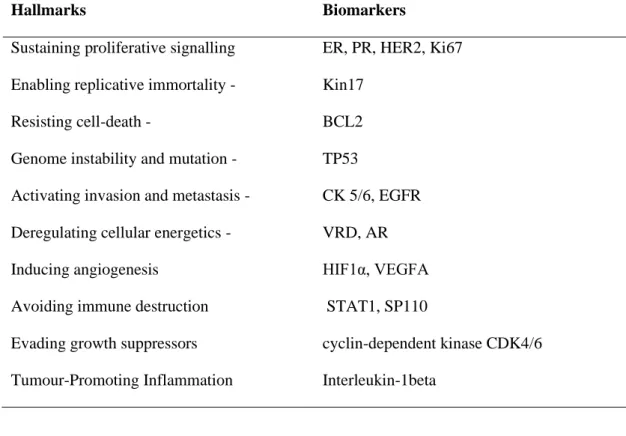
VGSCs: A common neuron hallmarks but now a new metastatic tumour marker