134 4.3.3 Association between dental anomalies in non-syndromic cleft lip with or without cleft palate (NSCL/P) and non-cleft children. 169 4.4.8 (a) Identification of significant copy number gain in TP73 (1p36.32) amon non-syndromic cleft lip with or without cleft palate (NSCL/P) non-cleft with hypodontia. 4.4.8 (b) Identification of significant copy number gain in SKI (1p36.33) among non-syndromic cleft lip with or without cleft palate (NSCL/P) and non-cleft with hypodontia.
4.4.8 (c) Identification of significant copy number gain in LEMD3 (12q14.3) between non-syndromic cleft lip with or without cleft palate (NSCL/P) and non-cleft with hypodontia. IGF1R (15q26.3) between non-syndromic cleft lip with or without cleft palate (NSCL/P) and non-cleft with. 4.4.8 (e) Identification of significant copy number loss in FHIT (3p14.2) between non-syndromic cleft lip with or without cleft palate (NSCL/P) and non-cleft with hypodontia.
4.4.8 (f) Identification of significant copy number loss in UGT2β15 (4q13.2) amon non-syndromic cleft lip with or without cleft palate (NSCL/P) and non-cleft with hypodontia. 206 4.5.3 (d) The association between loss of heterozygosity (LOH) of selected markers and hypodontia in non-syndromic cleft lip with or without cleft palate and non-cleft children.
SUMMARY AND CONCLUSIONS 245
124 Table 4.1 Sociodemographic characteristics of the study samples (n=161) 130 Table 4.2 Distribution of non-syndromic cleft lip with or without cleft.
PREVALEN ANOMALI PERGIGIAN DAN ABERASI GENETIK BAGI PESAKIT REKAHAN
BIBIR DENGAN ATAU TANPA REKAHAN LELANGIT BUKAN SINDROMIK YANG
MEMPUNYAI HIPODONSIA
Analisis data statistik dilakukan menggunakan ujian Mann-Whitney dan Chi-Square dalam perisian IBM SPSS untuk mengesahkan keputusan yang ketara. Analisis pengesahan mendedahkan peningkatan bilangan salinan yang ketara dalam TP73, SKI, IGF1R dan LEMD3 dan penurunan dalam FHIT dan UGT2β15 dalam kanak-kanak NSCL/P dengan hipodontia (p <0.005). Kesimpulannya, kajian ini telah berjaya mewujudkan kelaziman anomali pergigian dan mengenal pasti penyimpangan genetik di kalangan NSCL/P.
Kajian semasa menunjukkan penemuan baru kehilangan nombor salinan pada 3p14.2 dan 4q13.2 yang termasuk FHIT dan UGT2β15 yang mungkin menyumbang kepada pembentukan NSCL/P dengan hipodontia.
PREVALENCE OF DENTAL ANOMALIES AND GENETIC ABERRATIONS OF
NON-SYNDROMIC CLEFT-LIP WITH OR WITHOUT CLEFT PALATE PATIENTS WITH
HYPODONTIA
Research background
Nonsyndromic cleft lip with or without cleft palate (NSCL/P) is one of the most common congenital disabilities worldwide affecting the cleft lip and palate (Mohamad Shah et al., 2019). The incidence of clefts varies among populations through ethnic, racial, and geographic differences (Brito et al., 2011). Contributing environmental factors include maternal smoking, alcohol consumption, folic acid deficiency, and medication during pregnancy (Murray, 2002; . Lacerda et al., 2021).
It has been reported that patients with cleft lip and palate (CLP) show a higher frequency of dental anomalies than non-cleft subjects (Camporesi et al., 2010). Dental anomalies can be classified according to their number, size, shape, structural, developmental and positional characteristics (Akcam et al., 2010; Camporesi et al., 2010). The most common dental anomaly in cleft children is hypodontia, followed by supernumerary tooth (Camporesi et al., 2010).
The co-occurrence of cleft lip with or without cleft palate (CL/P) and hypodontia has been commonly reported in humans, which may parallel sharing or similar developmental mechanisms and genetic risks (Milien et al., 2016). According to the recommendations of the American Cleft Palate and Craniofacial Association, all individuals with craniofacial anomalies, including CL/P, should be treated by an interdisciplinary team of specialists (Watted et al., 2020). Increased risks of maternal smoking during the periconceptional period suggest that genes in specific metabolic pathways may play a role in developing NSCL/P (Huda et al., 2021).
Markers in the GSTT1 (glutathione S-transferase theta) or NOS3 (nitric oxide synthase 3) genes, in particular, appear to influence the risk of NSCL/P in the presence of maternal smoking (Shi et al., 2007). Hypodontia in patients with CL/P can significantly affect the patient's quality of life in terms of functional decline, emotional distress and social prosperity (Ghazali et al., 2021). CNV and LOH act by altering gene expression, disrupting gene sequences, and altering gene dosage ( Feuk et al., 2006 ).
Variations in the regulatory process of these transcription factors and gene mutations can alter the deoxyribonucleic acid (DNA) sequence, which accounts for many congenital abnormalities of the orofacial regions (Huda et al., 2021). Copy number variation has recently been identified as an important cause of structural genome variation that includes sequence duplications and deletions (Redon et al., 2006). A previous study identified copy number variations (CNVs) in patients with CL/P syndrome and hypodontia, such as ectodermal dysplasia syndrome, Wolf Hirschhorn syndrome, and DiGeorge syndrome (Phan et al., 2016).
Study rationale
Causative genes and genomic loci of hypodontia during syndromic CL/P may also lead to the development of non-syndromic CL/P. Therefore, genome-wide copy number analysis was performed to determine the contribution of CNVs in the development of NSCL/P with hypodontia. In addition, selected genes in the region of significant copy number gain and loss are subjected to a validation procedure using TaqMan copy number variation analysis.
This validation analysis examined the copy number of the gene of interest and normalized to an endogenous reference gene known to be present in two copies in a diploid genome between NSCL/P and uncleaved with hypodontia. This study also evaluated the associations between significant CNVs of selected markers and hypodontia in NSCL/P. The analysis of microsatellite markers included PCR amplification of the microsatellite loci at recurring selected markers using a fluorescently labeled primer flanking the repeated sequence.
Objectives
- General objective
- Specific objectives
Research questions
Research hypothesis
Cleft lip with or without cleft palate (CL/P)
Epidemiology of cleft lip with or without cleft palate (CL/P)
Embryological development of the cleft lip with or without cleft palate (CL/P)
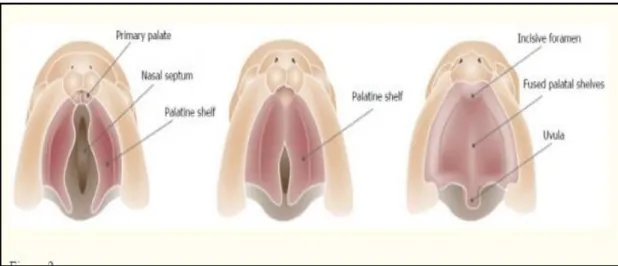
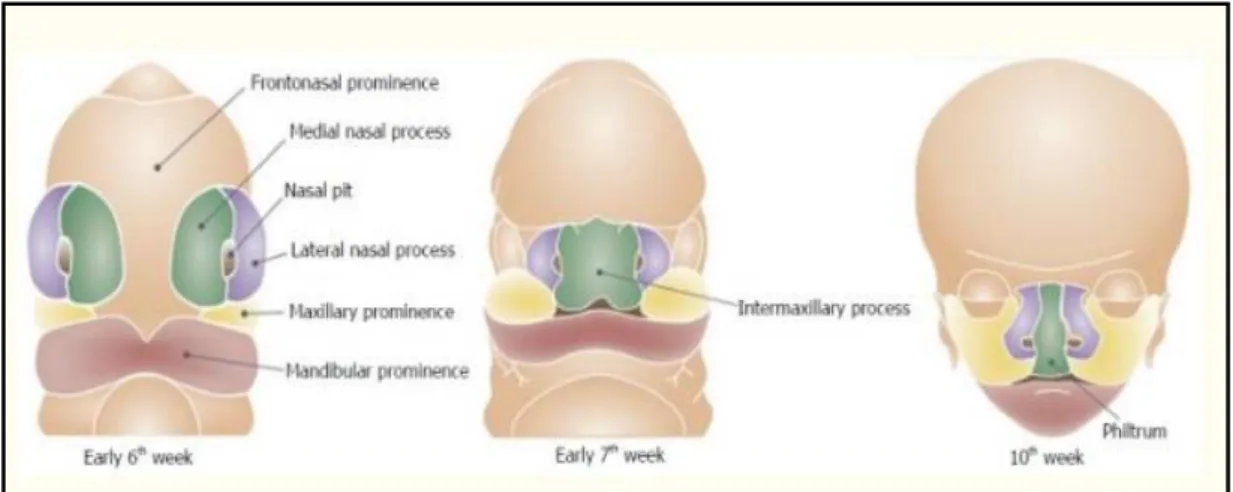
Figures 2.1 and 2.2 show respectively the development of the lip and caudal teeth of the palatal shelves' fusion process. When these processes do not mature or fuse, orofacial cleft affects the upper lip, alveolus, philtrum, incisors and primary palate (Leslie and Marazita, 2013; . Smarius et al., 2017). The primary palate is the ventral portion of the incisive foramen, while the secondary palate is dorsal to the incisive foramen.
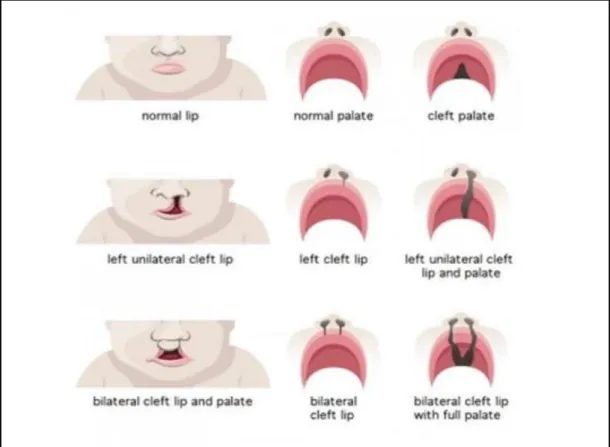
A cleft lip can be unilateral (on the left or right side) or bilateral (on both sides). A complete unilateral cleft lip is the result of a complete failure of fusion of the medial prominence of the nose and jaw on one side. A unilateral partial cleft lip is the result of partial medial nasal elevation and maxillary prominence on one side.
During the 7th week of embryogenesis, the secondary palate is typically developing two palatal shelves (PS) and growth from the maxillary prominence. The PS started in a vertical position that followed the edge of the tongue and then reconstructed to the horizontal position (Nakajima et al., 2018). Both the PS above the tongue contact in the horizontal position and the medial border epithelium (MEE) at the palatal midline sutures.
Cleft palate can develop as a result of a malfunction in different stages of the development of the secondary palate (Leslie and Marazita, 2013).
Types of Cleft
Adapted from bettersafercare.vic.gov.au/clinical-guidance/neonatal/cleft-lip-and-palate-in-neonates.
Etiology of non-syndromic cleft lip with or without cleft palate (NSCL/P)
- Genetic influences
- Environmental factors
The mutation on certain genes such as the TGFβ3, Apetala 2 (AP2) and MSX1 can change the signaling molecules, transcription factors or growth hormones in the growing prominences of the lip and palate and thus impair their natural fusion (Bender, 2000). A few triggers can cause cleft development, such as maternal smoking, maternal ingestion of pharmaceuticals such as anticonvulsants phenytoin and benzodiazepines or pesticides (Murray and Schutte, 2004). The discovery that folic acid supplementation can reduce the incidence of neural tube defects is one of the 21st century's significant genetics.
Tooth development
- Initiation stage
- Bud stage
- Cap stage
- Bell stage
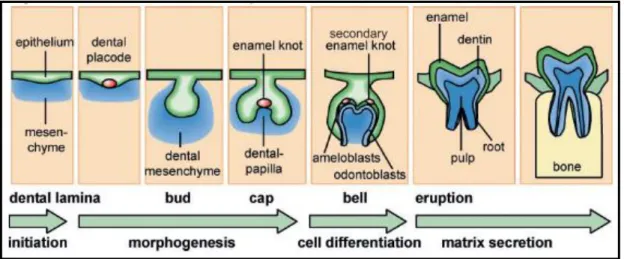
The oral epithelium thickens at the site of future tooth formation called the odontogenic zone or stomodeal epithelium. The dental placode consists of thickened epithelium and mesenchyme derived from the neural crest (Jussila and Thesleff, 2010.) The dental placode is the main epithelial structure of tooth formation and the first signaling center of the tooth. The stellate reticulum, a cluster of cells located in the center of the enamel organ, is found in the tooth bud (Waddington, 2009).
Disruptions in the bud phase can result in the formation of macrodontia and microdontia caused by excessive or insufficient cell proliferation. The cap stage of tooth development occurs between the 9th and 10th week of embryonic development. The enamel organ is formed by epithelial cells during the cap development stage, and the dental papilla is formed by mesenchymal cells.
This process is believed to be controlled by the primary enamel knot, which serves as a signaling center for the developing teeth (Vaahtokari et al., 1996). The primary enamel knot is formed during the transition from the bud to the cap stage and is fully differentiated at the beginning of the cap stage. Although enamel button cells do not multiply, they stimulate division of epithelial and mesenchymal cells (Catón and Tucker, 2009).
At the onset of the bell stage, primary enamel nodule cells die due to apoptosis and silencing of the signaling center. Cell differentiation occurs at the bell stage of development, and the ameloblasts and odontoblasts are established in the adjacent layer at the site of interaction between the epithelium and the mesenchyme.
Dental anomalies
- Alteration in number of teeth