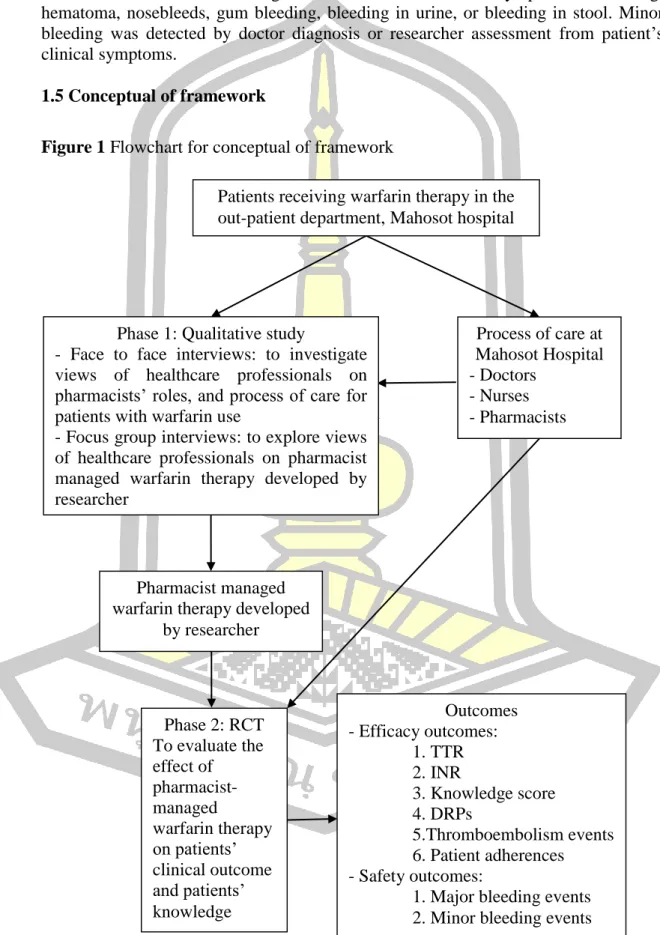
The results of the qualitative interviews were used to develop an RCT trial intervention called “pharmacist-managed warfarin therapy.” Conclusion: The time within the therapeutic range was statistically significantly higher in the intervention group than in the control group.
INTRODUCTION
- Background and rationale of the study
- Aim of the study
- The scope of the study
- Definition of specific terms
- Conceptual of framework
- Hypothesis
- Expected outcome and benefits
This study aims to establish and develop pharmacist-managed warfarin therapy at Mahosot Hospital, Lao PDR. Focus group interviews: to explore views of healthcare professionals about researcher-developed pharmacist-managed warfarin therapy.
LITERATURE REVIEW
- Introduction of warfarin
- Pharmacodynamics and pharmacokinetics of warfarin
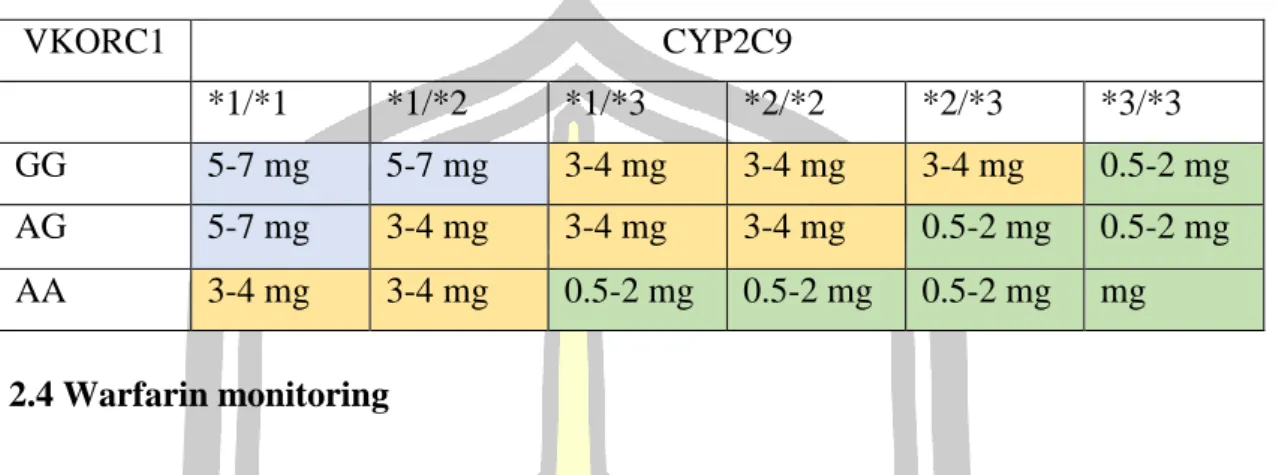
- Warfarin dosage
- Warfarin monitoring
- Drug related problems of warfarin therapy
- Nutritional factors and drug interaction on warfarin therapy
- Education for warfarin patients
- Literature reviews
- Pharmaceutical care practice
- Health care professional and patients’ perspective on warfarin therapy
- Current research of pharmacist managed warfarin therapy
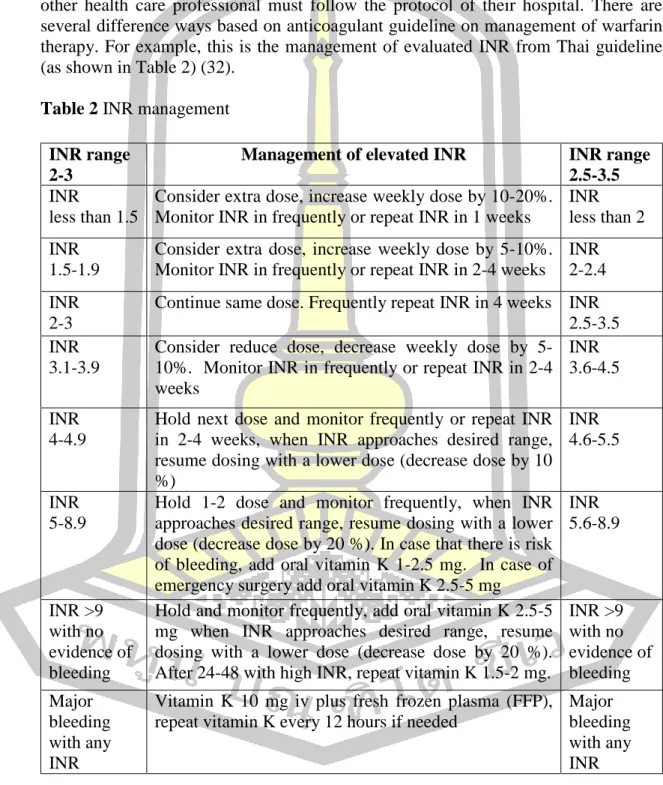
The annual incidence of major bleeding ranges from 1% to 10% depending on the quality of warfarin therapy management. In the intervention group, 73.45% of INR test results were within the therapeutic range during the 6-month data collection period.
METHODOLOGY
Phase 1: Qualitative interviews
- Research design
- Research setting
- Sample of study
- Research tool
- Validation of interview guides
- Recording tool
- Data collection procedures
- Data analysis
The researcher transcribed 9 interview tapes from face-to-face interviews and 8 interview tapes from focus group interviews. Several thematic frameworks were created based on themes identified from face-to-face and focus group interviews.
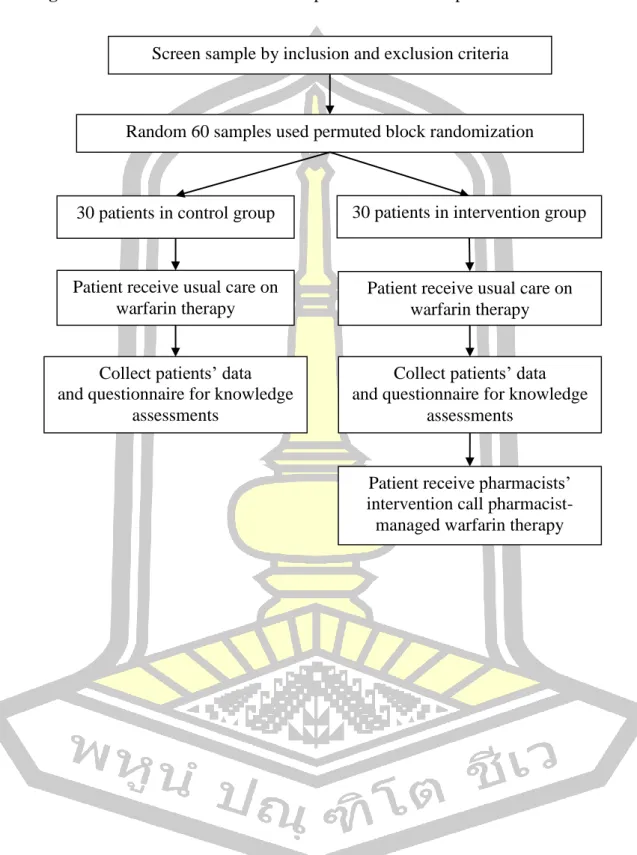
Phase 2: A Randomized Controlled Trial (RCT) study
- Research design
- Research setting
- Population and sample
- Inclusion criteria
- Exclusion criteria
- Sample size estimation
- Random sampling
- Research outcomes
- Measurements and data collection tool
- Quality of measurement instruments
- Data collection procedure
- Data analysis
Patients were asked a question following the warfarin therapy questionnaire to be patients' baseline knowledge. Patients were asked a question following the patients' knowledge assessment questionnaire to be patients' baseline knowledge.

Protection of human participants
Planning process
Therapeutic INR range of patients in the intervention group was 52.8% for patients with INR of 2-3 higher than 41.7% of patients in the control group. Total number of patients with target INR for all visits in the intervention group was compared with the control group. In addition, the mean INR value for patients in the intervention group was 2.2 ± 0.7 at the baseline visit.
In summary, there was no statistically significant difference between the mean INR at each visit for patients in the intervention group with a p value of 0.344. First, patients' knowledge was assessed for all 15 questions, the mean baseline score of patients in the intervention group was found to be 5.2 ± 2.6. Also, after one month the knowledge of patients in the intervention group increased again to 13.4 ± 2.1.
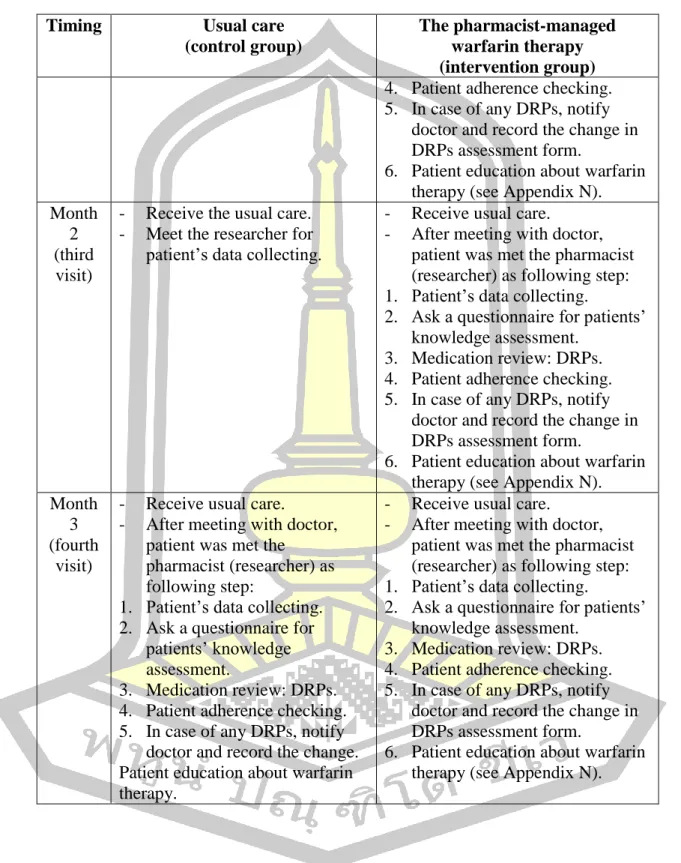
Thus, from the baseline visit, patients in the intervention group have lower mean knowledge scores than patients in the control group. The mean patient warfarin knowledge score in the control group was not statistically significantly different between baseline 3.3 ± 2.1 and third visit 3.5 ± 2.2. All patient care processes on pharmacist-managed warfarin therapy in this study were performed in the outpatient department.
Patients' comorbidities were most commonly found in hypertension: 22% of patients in the intervention group and 13.9% of patients in the control group. Willingness to participate in the study on the development of pharmacist-managed warfarin therapy in Mahosot Hospital, Lao PDR.

RESULTS
Qualitative interviews
Face to face semi-structured interviews
- Healthcare professionals’ experiences of current practice problems with
- Views of service and warfarin problems
- Roles of healthcare professionals in current practice
- Views of organizational barriers
- Healthcare professionals’ perspectives on ways to improve services and
- Ways to improve services
- Views of pharmacists’ roles in warfarin clinic
- Views of education or training and research
Focus group interviews
- General information of healthcare professional’s participant in focus
- Collaborations among healthcare professionals
- Expectations of pharmacists’ roles by healthcare professionals
- How to take warfarin properly
- Normal INR range
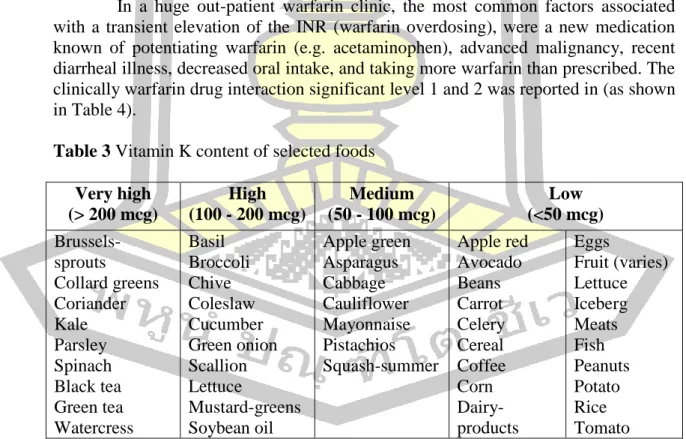
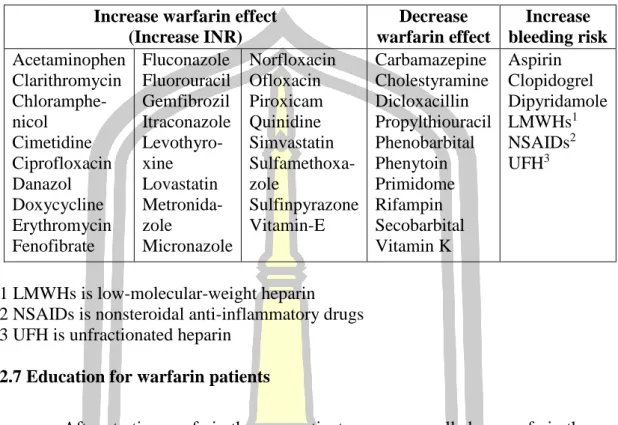
- Drug-, Food-, Herb-, Alcohol-warfarin interactions
- Adverse drug reaction and management
- How to manage when receiving operations
- Importance of following up doctors’ appointment
- Booklet information
- Development of training program for healthcare professionals
The face-to-face interviews were conducted to explore the views of healthcare professionals including doctors, nurses and pharmacists on warfarin therapy. Focus group interviews were undertaken to obtain views of health care professionals involved in the provision of health care services for patients using warfarin in order to develop the practical intervention called pharmacist-managed warfarin therapy at Mahosot Hospital integrated with evidence-based intervention model. The face-to-face interviews were conducted to explore opinions of healthcare professionals including doctors, nurses and pharmacists.
Three main themes emerged from the face-to-face interviews, which included views of warfarin services and problems, health professional roles in current practice and views of organizational barriers. It is very interesting that all health professionals agreed with pharmaceutical counseling for patients treated with warfarin. Focus group interviews were conducted to obtain the views of health professionals involved in the provision of health services for patients using warfarin.
The care process for “pharmacist-managed warfarin therapy” was then established and discussed in the focus group interviews. Furthermore, the summaries of a personal interview with healthcare professionals at Mahosot Hospital presented two main themes: healthcare professionals. A total of 8 healthcare professionals working at the outpatient clinic were interviewed (2 doctors, 3 nurses and 3 pharmacists).
A Randomized Controlled Trial Study
Patient characteristics outcomes
Efficacy outcomes
- Time in therapeutic range (TTR)
- INR value
- Patients’ knowledge
- Drug-related problems (DRPs) with warfarin therapy
- Thromboembolism event
- Patients’ adherences
Safety outcomes
- Major bleeding
- Minor bleeding
INR in therapeutic range in the intervention group was higher than the percentage of patients in the control group 73.7% with 61.7. But there was a statistically significant difference between the mean INR in each visit for the patient in the control group (p-value = 0.011). But the third visit, after being advised by pharmacist 3 times, the patients' knowledge of the intervention group was higher than the control group patients with a statistically significant difference between two groups (p-value = 0.001).
The result also showed that DRPs decreased from baseline to the first visit in the intervention group (20 DRPs to 6 DRPs). This study found that the percentage of patients with INR within the therapeutic range in the intervention group was higher than the percentage of patients in the control group (73.7% and 61.7%, respectively). During the study, minor bleeding was still found at the first visit in 2 cases in the intervention group.
Characteristic of our study, the mean age was years old for patients in the intervention group and years old in the control group.
CONCLUSION, DISCUSSION and LIMITATION
Conclusion
A semi-structured, face-to-face interview was conducted to find out healthcare professionals' views on pharmacists' roles and treatment process for patients with warfarin use. Two main themes emerged from the face-to-face interviews, consisting of (1) healthcare professionals' experiences of current practice issues with warfarin therapy; and (2) health professionals' perspectives on ways to improve services and health professionals' education and training. The major themes that emerged during the focus group interviews included (1) collaborations between health professionals (2) health professionals' expectations of pharmacists' roles, and (3) development of training programs for health professionals.
By analyzing the TTR of 72 patients enrolled in our study, the results of TTR for patients in the intervention group and for patients in the control group, with statistically significant difference between two groups (p-value = 0.046). The most type of DRPs from 4 visits was sub-therapeutic dose consisting of 30 cases in the intervention group (12, 6, 6 and 6 cases at baseline, first, second and third visit, respectively). For the baseline visit, patient compliance in the intervention group was 69.4% lower than other visits, followed by 97.2% at the first, 100.0% at the second and third visits.
Nevertheless, there were 88.9% of patients in the control group who achieved good adherence at the baseline visit.
Discussion
- Qualitative study
- RCT study
Additionally, the percentage of time patients' INR values were within the extended therapeutic range was 77.3% versus 67.3%. There was also a strong focus on patient education as part of this intervention, as studies have generally shown an association between patient knowledge of warfarin therapy between intervention and control groups. When pharmacists participated in routine warfarin knowledge and patient behavior care, patients would have better warfarin use, as evidenced by TTR being higher in the intervention group.
Hasan and colleagues focused on patients' knowledge scores about warfarin's mechanism of action, the interaction between warfarin and alcohol, and knowledge about warfarin's side effects. Also for patients' adherence assessed by the MGL 4-item scale, the results showed that patients in both groups had good adherence as at the first, second and third visits. To solve patients' adherence, we need to better understand and gain more knowledge about warfarin treatment.
For minor bleeding there is a chance of improvement event the patients' INR in therapeutic range.
Limitation of study
Since the similarity between the questionnaire 4 points and 8 points, the result showed good patient compliance, contrary to pill number assessment method. Kimmel and colleagues (72) investigated a prospective cohort study in 3 anticoagulation clinics to determine the effect of adherence on anticoagulation control. Tang and colleagues reported that patients with better understanding and knowledge of warfarin therapy had better therapeutic control (73).
Bungard et al and Chan et al (46, 60) found that bleeding was still reported in the pharmacist group (2 patients and 1 patient, respectively) when pharmacists were involved in dose adjustments. The only way to check or know the patient's record was the patient's book, which they bring home every time and can sometimes forget it at home when they come for a check-up. Due to the lack of patient records, the researcher could not be sure of the number of all warfarin patients, the number of patients monitored at each visit.
The longer follow-up was recommended to better understand patients' understanding, adherence, efficacy and safety outcomes, and any issues caused by warfarin regarding the information provided.
Future research
Furthermore, there were no records of ADRs or DRPs of warfarin, which we recommended future study to continue collecting these data for the baseline and reduce the number of missing patients admitted. The number of patients included in the study was very small, so it was difficult to compare the results of the two groups. There were only 36 people in each group which was very difficult to find thromboembolism and major bleeding events.
If we could have more patients, we could increase the chance of finding thromboembolism event and major bleeding. This may be due to the time-consuming and the difference in compliance or follow-up that may affect the effectiveness and safety of pharmacist-managed warfarin therapy.
Application in clinical practice
If you are in the case of a focus group interview, 4 hours will be used in the meeting. If you are not protected as shown in the data explanations of the participant information sheet in the study. I know the right to get more information about both the benefits and penalties of participating in the research.
I can stop participating in the research at any time without losing any of my rights as a patient here. Please report the abnormal symptoms that occurred to you during your participation in the research project. So please inform the researcher about the drugs you received while you were in the research project.
Please provide the researcher with your past and present medical information truthfully. Please inform the researcher of any irregularities that occurred while you participated in the research project. If you do not voluntarily participate in the study, you can withdraw at any time.
DRPs assessment form