This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering. I would also like to acknowledge all members of the Department of Chemical and Petroleum Engineering at the UAE University for their kind assistance during my studies.
Introduction
Statement of the Problem
Research Objectives
Organization of Thesis
Literature Review
Biodiesel Production
Conventional Catalysts
In contrast, the use of KOH alone under the same reaction conditions gave a biodiesel yield of 94.9% [42]. To overcome the disadvantages of homogeneous catalysts, heterogeneous solid catalysts have been used in many organic reactions [43] as they can reduce soap formation and can be easily separated and reused.
Enzymatic Biodiesel Production
- Factors Affecting Enzymatic Biodiesel Production
Therefore, an organic solvent is usually added to increase the solubility of substrates and reduce the inhibitory effect of methanol [78]. The addition of an organic solvent can also reduce the viscosity of the reaction medium and increase the stability and yield of the immobilized enzyme [79].
Immobilized Enzymes in Biodiesel Production
Thus, an outer glycerol film is formed, which reduces the mass transfer of hydrophobic substrates to the active sites of the enzyme, leading to lower reaction rates [71]. Furthermore, enzyme immobilization is highly dependent on the amine functional group of amino acids in the enzyme, which contribute to binding to the support.
MOF’s
- MOF Structure and Properties
- MOF Preparation
- Properties of MOF-Immobilized Enzymes
For example, the residual activity of lipase@ZIF-8 after repeated use for seven cycles was 54% of that in the first cycle, while after storage for 25 days it was 90% of the initial activity [109]. However, the nano-size of enzyme-MOF conjugates hinders their reuse on an industrial scale.
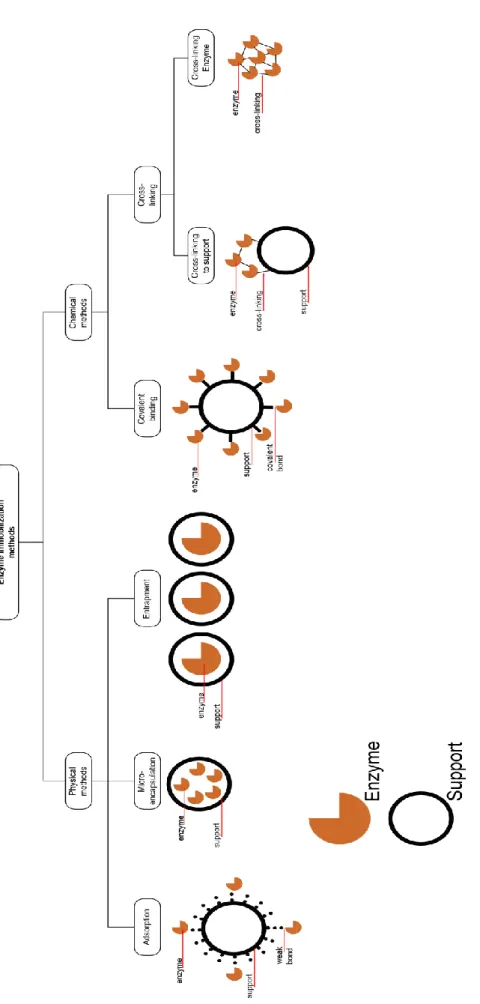
Lipase Immobilization on MOF’s
- Physical Adsorption
- Covalent Binding
- Entrapment/Encapsulation
- Cross-Linking
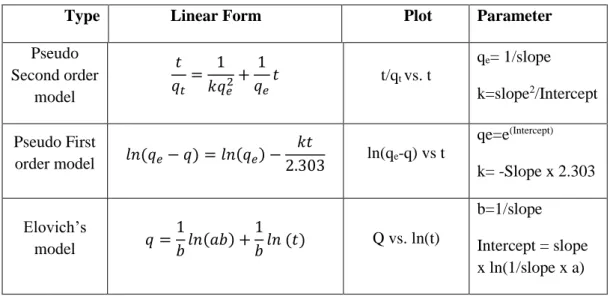
Another method that has recently been developed to overcome the enzyme interaction problem with the de novo MOF approach. The interactions between the enzyme and the MOF have marginal influence on the de novo system, which has a positive effect on the enzyme activity.
Use of Immobilized Enzyme in Biodiesel Production
The biodiesel yield from soybean oil using Novozym@435 in [Emim][TfO] IL was 80% after 6 h, which was eight times higher than that archived in solvent-free system and 15% higher than using tert-butanol as an additive [133] under the same conditions. By using Rhizomucor miehei lipase encapsulated in X-shaped ZIF-8, a biodiesel production conversion of soybean oil reached 92.3%. Novozym 435 was recycled 10 times, with the conversion reaching 88% and 65% after the 11th reaction with ethanol and methanol, respectively [137].
When Novozym 435 was used in the esterification reaction in the presence of IL [BMIM][PF6] and methyl acetate as acyl acceptor, a biodiesel yield of 80% was obtained at the optimum conditions with a molar ratio of 14:1 oil:acyl acceptor; 20%. w immobilized lipase/weight oil) and a temperature in the range 48-55°C. A covalently immobilized Candida antarctica lipase (CALB) on the bio-based MOF with adenine as the organic ligand based on the concept of biomimetic assembly was used for the esterification of oleic acid with methanol for biodiesel production.
Cost Analysis and Viability of Immobilized Enzymes
The successful immobilization of the enzyme on supports for sustained activity and stability, with minimization of mass transfer limitation, plays a major role in shifting the economic balance toward the enzymatic process. The main challenges are the high cost of the carrier support, low enzyme capacity, and challenges maintaining enzymatic activity and stability. In addition, the advances in biotechnology promise to offer new enzymes with lower production costs and higher catalytic activity and stability, which will further improve the feasibility of enzymatic process.
Among the most promising alternative solvents are ILs, which as mentioned earlier can further enhance enzyme reusability by enhanced mass transfer [123]. The cost of the most commonly used ILs in enzymatic biodiesel production, namely 1-n-butyl-3-methylimidazolium tetrafluoroborate ([BMI][BF4]) and 1-n-butyl-3-methylimidazolium hexafluorophosphate ([BMI][PF6 ]). ), are about 25 times more than organic solvents.
Hypothesis
The properties of DES can be fine-tuned, similar to ILs, by choosing different combinations of cations and anions. They have similar characteristics to ILs, such as high purity, easy preparation, non-toxicity, biodegradability, require mild reaction conditions and insensitivity to water [146]. However, with advances in raw material selection and synthesis technology, lower prices comparable to those of synthetic zeolites can be achieved for the large-scale synthesis of some MOFs in the future [147]. production and for future improvement), high biodiesel production (Hypothesis:.
Due to the high surface area of the MOF to immobilize the enzyme, a much lower mass transfer can be achieved compared to conventional immobilized lipase and allows its repeated reuse).
Methods
Method and Theory
As an alternative is chemisorption, where the nucleophiles of the enzymes (free amino acids) are covalently bound to the organic linkers (mainly carboxylate groups) of MOFs to form peptide bonds [163]. In this case, the enzyme is caged inside the pores of the MOFs during the crystallization process. Kinetics and thermodynamic studies of the adoption process, which are scarce in the literature, could provide invaluable information on surface chemical affinity, enzyme accessibility, and leaching [149] .
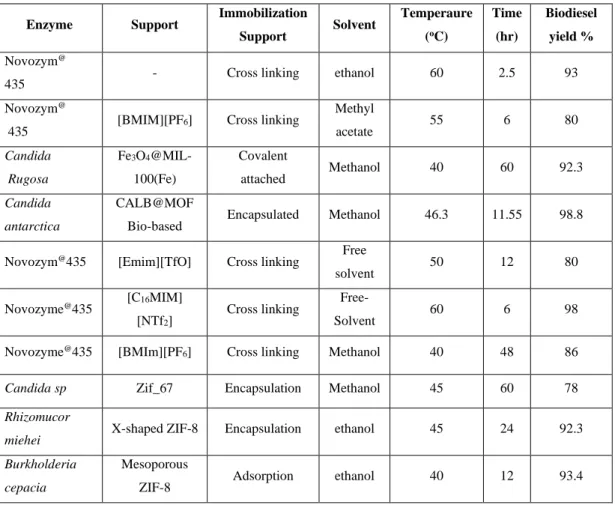
The Langmuir isotherm assumes uniform and constant binding of the sorbate on the surface of the adsorbent. The Freundlich model does not have a thermodynamic basis and does not offer a physical interpretation of the adsorption data.
Materials
MOFs Synthesis
- Synthesis of ZIF-67
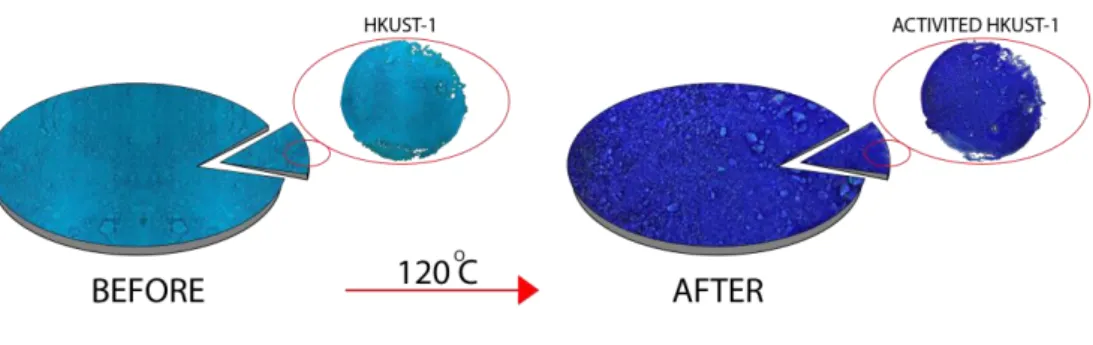
- Synthesis of HKUST-1
- Synthesis of Hexahedral Mesoporous ZIF-8
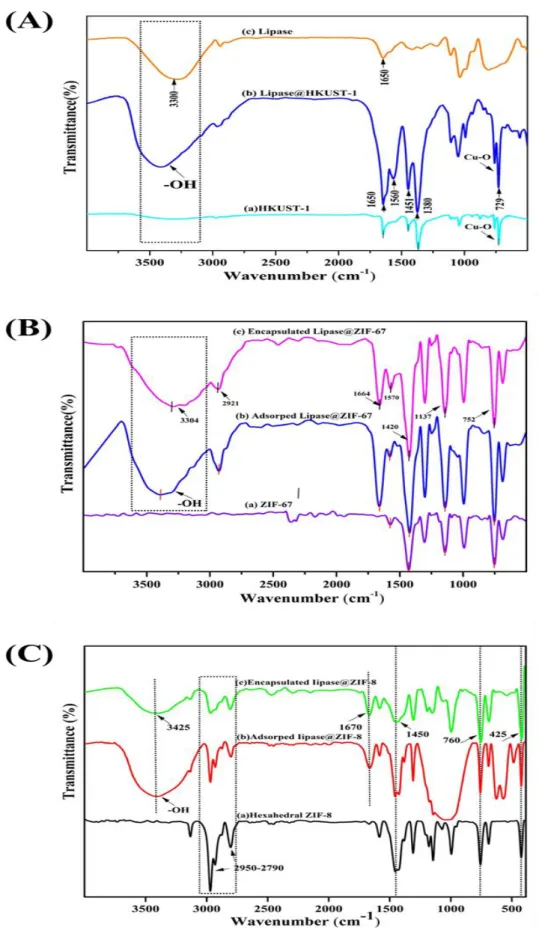
The surface morphology of the materials was observed with FE-SEM (Fei Apreo C, Czech Republic). The characteristic peak at 1380 cm-1 in Fig. 21A(a) is assigned to C–O BTC, and the bands at 1451 cm-1 and 1560 cm-1 were assigned to C=O BTC. The characteristic peak at 1650 cm-1, which is due to the aromatic C=C BTC, indicates the stretching vibration of the carboxylate group). The blank ZIF-67 pattern in Fig. 22B(b) is similar to those reported in the literature [179] and the simulated crystal shown in Fig. 22B(a), confirming the successful synthesis of ZIF-67 (CIF: 7222297). ).
In the initial phase of the adsorption process, the film diffusion is an important rate-controlling step [156]. The rate-limiting step in the adsorption process was found to be affected by intraparticle diffusion for ZIF-8 and ZIF-67.
Batch Adsorption of Lipase on Activated MOFs
Biodiesel Production
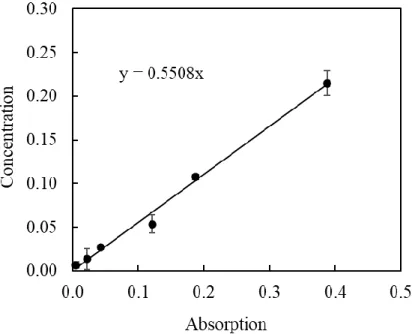
For biodiesel production experiment, the immobilized enzyme in MOFs (both encapsulated and adsorbed) was used at the optimum condition as found in Section 2.3. The reaction mixture consisted of 1 g of olive oil and 0.57 mL of methanol (equivalent to 1:12 molar ratio), and 1 mL of n-hexane-based or preliminary test. The experiments were performed in duplicate, and the results presented are the mean values, with the standard deviation as error bars shown in the figures.
At the end of the reaction described above, the immobilized enzyme was separated by centrifugation at 8600 rpm and used again in another procedure with fresh oil, methanol and hexane. Methanol concentration and enzyme loading were constant at 0.057 mol/mL and 0.02 mg/mL, while oil concentration was varied in the range of 0.5-0.3 mg/mL.
MOF Characterization
To model the diffusion reaction system using lipase adsorbed on ZIF-8, the experiment was carried out using different initial oil concentrations, keeping the concentration of methanol and enzyme consistent, adjusting the volume of n-hexane to bring the vol. total in 10. mL. Assuming the production rate to be linear in the first 4 h of reaction, the initial rate of reaction in each condition was calculated by dividing the concentration of FAMEs after 4 h of reaction by 4 h. Hydrophobicity of ZIF-8, ZIF-67 and HKUST-1 was tested using contact angle measurements using Rame-hart Instrument co at 110-240VAC.
The image of water falling on the surface was captured and the contact angles were determined by drawing a tangent near the edge of the drop. The reported contact angle values were the average of three separate measurements taken at three different locations on the surface.
Results
MOFs Characterization
- FTIR
- XRD
- Raman Spectroscopy
- Porosity and Surface Area
- Surface Hydrophobicity
- Surface Morphology
The above bands were in the spectra of ZIF-8, with lipase adsorbed and encapsulated, shown in Figures 21C(b) and 21C(c). The intensity of the lipase adsorbed peaks decreased slightly compared to the empty crystals, indicating that lipase adsorption did not affect crystallinity. Lipase adsorption preserved the morphology of ZIF-67 and the attachment of the enzyme did not affect the crystal structures.
However, we observed a decrease in crystal size, which indicates that the presence of the enzyme molecule during crystallization influenced the process. The decrease in ZIF-8 crystal size after lipase encapsulation was greater than that observed for ZIF-67.
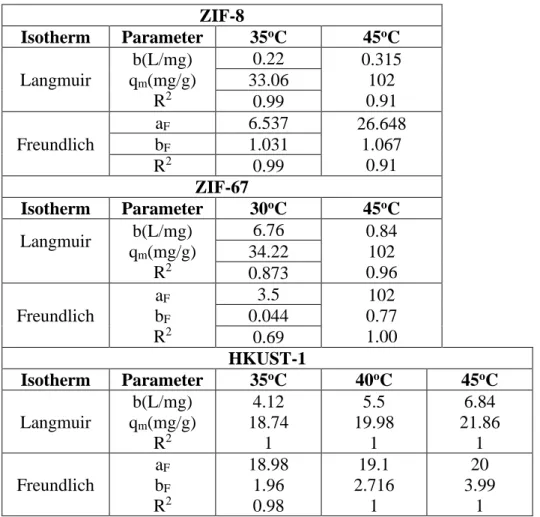
Lipase Adsorption Isotherms
From the Langmuir fit, it was found that the maximum adsorption capacity of ZIF-8, ZIF-67 and HKUST-1 increased with temperature, which is typical for the adsorption of most proteins [152]. The value of b, which is indicative of adsorbate-adsorbent interaction [191] increased with temperature for ZIF-8 and HKUST-1 (i.e., endothermic process), but decreased for ZIF-67 (i.e., exothermic. As shown in table 6, positive values of ∆G were obtained at all tested temperatures, except for ZIF-67 at the lowest tested temperature of 30oC and HKUST-1 at the highest tested temperature of 45oC.
Whereas for ZIF-67 and HKUST-1 the process becomes spontaneous at the low and high temperatures, respectively. The binding of lipase on ZIF-8 and ZIF-67 is by physical adsorption, where it is by chemical adsorption for HKUST-1.
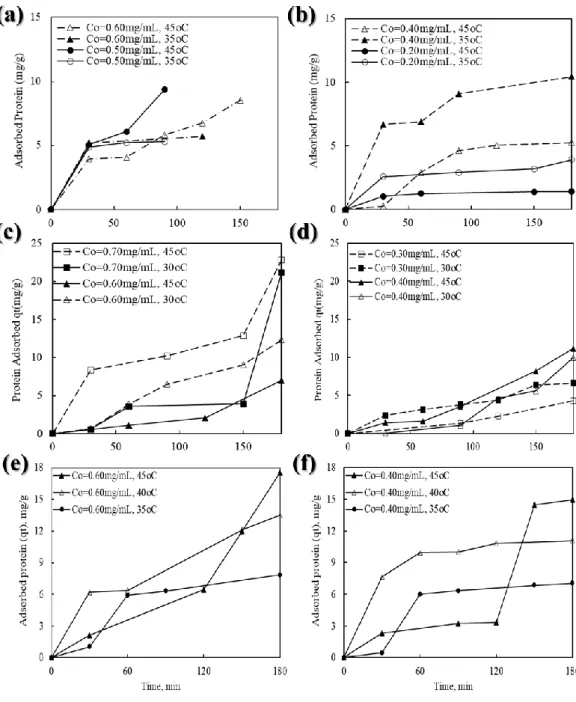
Adsorption Kinetics
12:1 methanol: oil ratio and 4 h (a) biodiesel production yield % by Adsorbed Lipase on ZIF-67, ZIF-8 and HKUST-1 (b) relative activity of biodiesel production using adsorbed enzyme. SiO2 Beads Decorated with SrO Nanoparticles for Biodiesel Production from Waste Cooking Oil Using Microwave Irradiation. One-step biodiesel production from waste cooking oils over metal-incorporated MCM-41; The positive effect of the template.
Efficient production of biodiesel from oleic acid using metal-organic framework encapsulated Zr-doped polyoxometalate nanohybrids. Efficient production of biodiesel from oleic acid using metal-organic framework encapsulated ZR-doped polyoxometalate nano-hybrids.
Encapsulation of lipase on ZIF-67 and ZIF-8
Transesterification Reaction and Operational Stability
Despite the adsorbed case, the activity of encapsulated lipase in ZIF-67 was lower than that in ZIF-8 and lower than that of adsorbed lipase in ZIF-67. Despite the higher stability of encapsulated lipase compared to adsorbed lipase, the continued decrease in activity suggests that a high percentage of lipase was actually attached to the outer surface due to the large surface area of ZIF-67. This suggests that a higher percentage of the enzyme was attached to the outer surface of ZIF-8, compared to ZIF-67, which was prone to leaching.
Assuming that the entire surface is equally accessible, the rate of substrate flow to the surface can be expressed by Eq. The higher the value of , the greater the diffusion resistance compared to the surface reaction resistance.

Future work
Conclusion
A comparative study of solid carbonic acid catalysts for the esterification of free fatty acids for biodiesel production. Biodiesel production from waste cooking oils through esterification: Catalyst control, chemical equilibrium and reaction kinetics. Intensification of one-step biodiesel production from high-water cooking oils and free fatty acid residues.
Comparative analysis of the effect of methanol and ethanol on Karanja biodiesel production and its optimization. A comparative study between chemical and enzymatic transesterification of high free fatty acid contained rubber seed oil for biodiesel production.