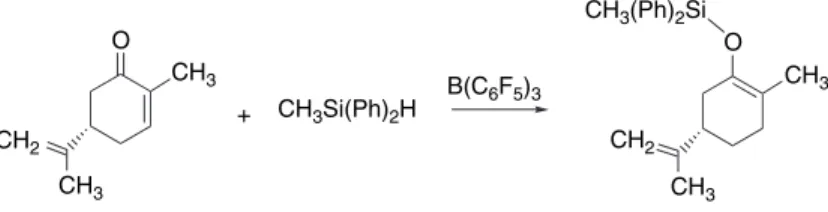The carbon-carbon bond forming reactions are highlighted and the stereoselectivity of the reactions is discussed in detail. The reactions in this chapter are among the most important and most common of the carbon-carbon bond-forming reactions.
Reactions of Carbon Nucleophiles
Functional Group Interconversion
Reduction of Carbon-Carbon Multiple Bonds, Carbonyl
Concerted Cycloadditions, Unimolecular Rearrangements,
Generation and Properties of Enolates and Other Stabilized Carbanions
- Generation of Enolates by Deprotonation
- Regioselectivity and Stereoselectivity in Enolate Formation from Ketones and Esters
Fundamental aspects of the structure and stability of carbanions were discussed in Chapter 6 of Part A. The acidity of the reactant determines which bases can be used to form the anion.
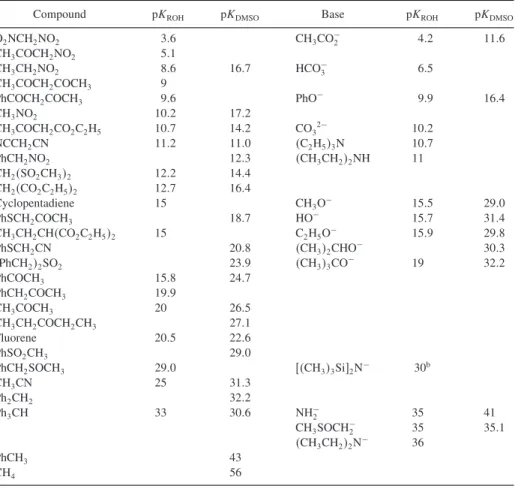
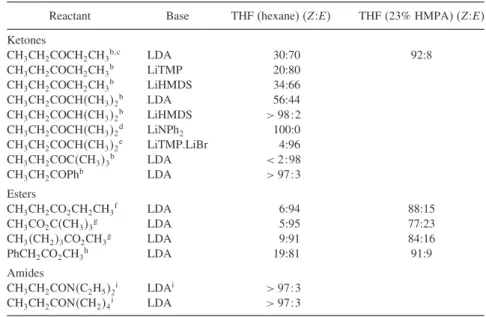
Composition of Enolate Mixtures Formed under Kinetic and Thermodynamic Control a
Other Means of Generating Enolates
Cleavage of trimethylsilyl enol ethers or enol acetates by methyllithium (entry 1 and 3), depends on the availability of these materials in high purity. Trimethylsilyl enol ethers can also be cleaved by tetraalkylammonium fluoride (entry 2). The driving force for this reaction is the formation of the very strong Si−F bond, which has a binding energy of 142 kcal/mol.31 These conditions also lead to equilibrium.
Other Means of Generating Specific Enolates
Solvent Effects on Enolate Structure and Reactivity
The inclusion of HMPA improved the C(1) selectivity to 11:1 and also markedly accelerated the rate of the reaction. The tighter coordination reduces the reactivity of the enolate and gives rise to more highly associated species.
Alkylation of Enolates 47
- Alkylation of Highly Stabilized Enolates
The smaller, harder Mg2+ and Li+ cations are more closely associated with the enolate than the Na+ and K+ ions. Note also the stereoselectivity in entry 7, where the existing branched substituent leads to a transorientation of the methyl group.
Alkylation of Enolates Stabilized by Two Functional Groups
Alkylation of Ketone Enolates
The top face of the enolate presents three hydrogens in a 1,3-diaxial relationship with the approaching electrophile. Axial attack from the lower part of the face leads directly to the conformation of the product seat.
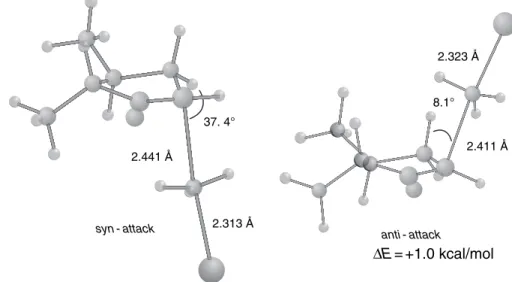
Alkylation of Ketone Enolates
Alkylation of Aldehydes, Esters, Carboxylic Acids, Amides, and Nitriles Among the compounds capable of forming enolates, the alkylation of ketones
A careful study of the alkylation of various enolates of dialkyl malate esters has been reported.74 These esters form dianions resulting from deprotonation of the hydroxyl group. This result can be understood by considering the differences in the faces of the enolates.
Generation and Alkylation of Dianions
Intramolecular Alkylation of Enolates
Generation and Alkylation of Dianions
Geometrical factors in the TS are also responsible for differences in the case of cyclization of enolates9 and 10.84. The reduced selectivity of the 5-methyl isomer may be due to the fact that the methyl group is further away from the reaction site than in the other cases. An intramolecular alkylation following this stereochemical pattern was used in the synthesis of (-)-fumagillol, with the alkadienyl substituent exerting the dominant conformational effect.88.
Intramolecular Enolate Alkylation
Control of Enantioselectivity in Alkylation Reactions
In enantioselective synthesis, it is necessary to control the direction of approach and thus the configuration of the new stereocenter. As with the acyloxazolidinone auxiliaries, each of these systems allows for hydrolytic removal and recovery of the chiral auxiliaries. When two successive alkylations are done, both groups are added from the endo face so that the configuration of the newly formed quaternary center can be controlled.
The Nitrogen Analogs of Enols and Enolates: Enamines and Imine Anions
The nucleophilicity of carbon atoms enables the synthetic use of enamines for alkylation reactions. The conjugation between the nitrogen atom and the double bond teorbitals favors the coplanarity of the bonds, which are obscured in the structures. Due to the predominance of the less substituted enamine, alkylation occurs mainly at the less substituted -carbon.
Alkylation of Enamines
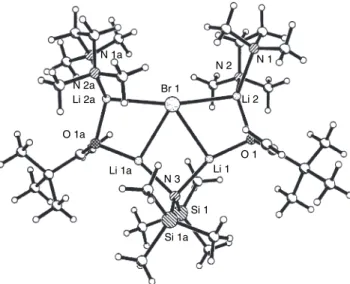
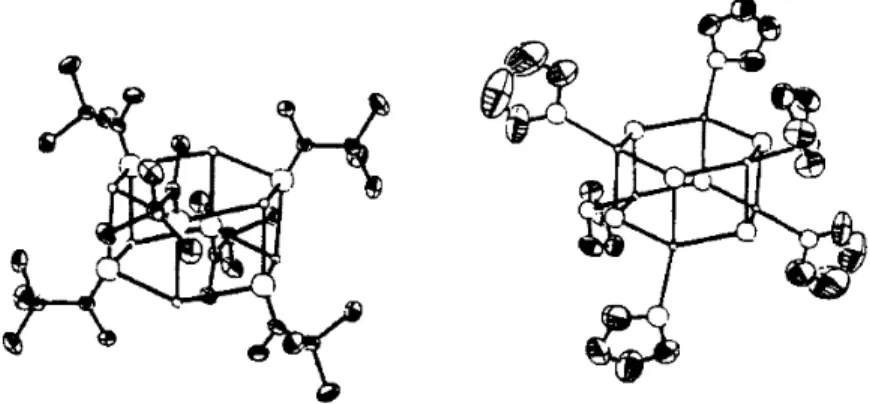
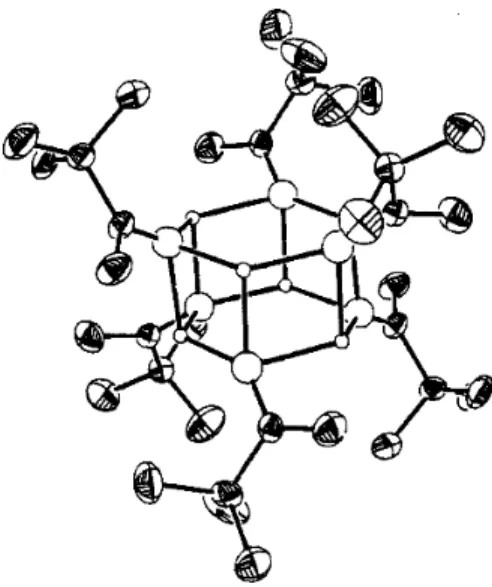
One of the complicating factors is that there are two imine stereoisomers, each of which can give rise to two regioisomeric imine anions. One of the potentially most useful aspects of the imine anions is that they can be prepared from enantiomerically pure amines. The crystal structure of the lithium anion of the SAMP hydrazone from 2-acetylnaphthalene has been determined126 (Figure 1.7).
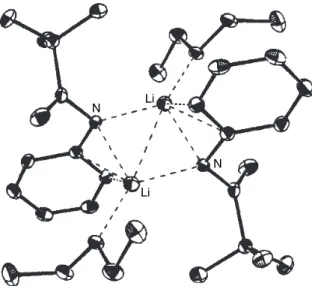
Alkylation of Imine and Hydrazone Anions
Aldol Addition and Condensation Reactions
- The General Mechanism
- Control of Regio- and Stereoselectivity of Aldol Reactions of Aldehydes and Ketones
General mechanistic features of addition and aldol condensation reactions of aldehydes and ketones were discussed in section 7.7 of part A, where these general mechanisms can be reviewed. The synthetic utility of the aldol reaction depends both on the versatility of the reactants and on the control of the region and stereochemistry. The reaction is under kinetic control, both in the enolate formation phase and in the addition phase.
Examples of Directed Aldol Reactions
Aldol Addition Reactions of Enolates of Esters and Other Carbonyl Derivatives
Inclusion of a strong cation-solvating co-solvent, such as HMPA or DMPU, favors the Z-enolate.36 These enolates can be trapped and analyzed as the corresponding silyl ketene acetals. Borenolates can be obtained from esters40 41 and amides42 by methods similar to those used for ketones. Among the most useful carbonyl derivatives are N-acyloxazolidinones, and as we shall see in section 2.3.4, they provide facial selectivity in aldol addition reactions.
The Mukaiyama Aldol Reaction
Trialkylsilyl cations can play a key role in other Lewis acid-catalyzed reactions.59 For example, trimethylsilyl triflate can be formed by intermolecular transfer of the silyl group. Bu2SnO3SCF32.68 The Lewis acids promote ionization of the acetal to an oxonium ion which acts as the electrophile. In some cases the enolate can be formed directly in the presence of the acetal with the Lewis acid also activating the acetal.69.
Control of Facial Selectivity in Aldol and Mukaiyama Aldol Reactions In the discussion of the stereochemistry of aldol and Mukaiyama reactions,
Stereoselectivity issues are the same as in the aldol addition reaction, but the trend toward acyclic rather than cyclic TSs reduces the influence of the E- or Z-configuration of the enolate equivalent on stereoselectivity. Interestingly, Entry 10 shows much reduced stereoselectivity compared to the corresponding aldehyde reaction (The BF3-catalyzed reaction of the aldehyde is reported to be 24:1 in favor of the anti product; ref. 80, p. 91). Control of Face Selectivity in Aldol and Mukaiyama Aldol ReactionsIn discussing the stereochemistry of aldol and Mukaiyama reactions,.
The Mukaiyama Aldol Reaction
Continued)
The stereoselectivity depends on the orientation of the stereocenter relative to the rest of the TS. This TS is preferred because of the interaction between the RM group and the R2 group of the enolate. The reaction shows a 13:1 preference for the Felkin approximate product (3,4-syn) and is controlled by the steric effect of the -methyl substituent.
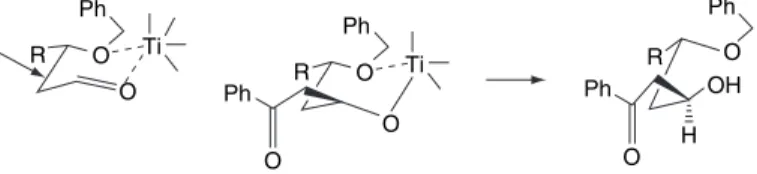
Examples of Aldol and Mukaiyama Reactions with Stereoselectivity Based on Aldehyde Structure
Continued)
The nonchelating boron is thought to react through a TS in which the conformation of the substituent is controlled by a dipolar effect. An indication of the relative effectiveness of oxygen substituent in promoting chelation of lithium enolates is found in the enolates3a–d. This effect is seen in the case of ketone4, where the stereoselectivity of the benzyloxy derivative is much higher than the compound lacking the benzyloxy group.110.
Examples of Facial Selectivity in Aldol and Mukaiyama Reactions Based on Enolate Structure
For example, the ketone 8 and the four stereoisomers of the aldehyde 9 have been examined.117 Both E-boron and Z-titanium enolates were studied. Corresponding selectivity ratios have also been determined for lithium enolates.118 Comparison with boron enolates shows that although the stereoselectivity of the fully matched system is higher with the boron enolate, in mismatched cases for the lithium enolate, the aldehyde bias exceeds the enolate bias and gives modest selectivity for the alternative anti-isomer. The stereochemistry of the -TBDMSO group in the aldehyde has little effect on the stereoselectivity.
Examples of Double Stereodifferentiation in Aldol and Mukaiyama Reactions
Continued)
The choice of reactant and conditions can be used to exert a considerable degree of control over stereoselectivity. The face selectivity in these chiral boron enolates originates from the steric effects of the boron substituents. The 2,3 synrelation arises from the Z configuration of the enolate and the 3,4 anti-stereochemistry is determined by the stereocenters in the aldehyde.
Control of Stereochemistry of Aldol and Mukaiyama Aldol Reactions Using Chiral Auxiliaries
Continued)
It was suggested that the excess reagent or silver salt removes a Cl- from the titanium coordination sphere and promotes chelation with the thion sulfur.142 This alters the face selectivity of the enolate by causing a reorientation of the oxazolidinethione ring. This often allows control of synorant stereoselectivity.143 The boron enolates give syn products, but the incorporation of SnCl4 or TiCl4 gave excellent selectivity for antiproducts and high enantioselectivity for a range of aldehydes.145. In the case of boron enolates of the camphor sulfonamides, the TiCl4-mediated reaction is believed to proceed via an open TS, while the reaction in the absence proceeds via a cyclic TS.
Examples of Control of Stereoselectivity by Use of Additional Lewis Acid
An interesting example of the application of this type of catalysis is a case where the addition reaction of 3-methylcyclohex-2-enone to 5-methyl-2-hexenal was studied over a range of conditions. Moreover, as this particular case also shows, they can be highly dependent on the specific structure of the catalyst. The structure of the active catalyst and the mechanism of catalysis have not been fully defined.
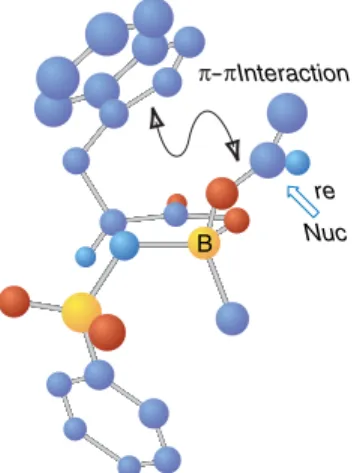
Chiral Catalysts for the Mukaiyama Aldol Reactions
Enantioselective Catalysis of Aldol and Mukaiyama Aldol Reactions
Intramolecular Aldol Reactions and the Robinson Annulation
Kinetic studies on cyclization of 5-oxohexanal, 2,5-hexanedione, and 2,6-heptanedione indicate that five-membered ring formation is thermodynamically slightly more favorable than six-membered ring formation, but the latter several thousand times faster.170 A catalytic amount acid or base is often sufficient to form five and six rings, but more complex structures use the techniques required for directed aldol condensations. The regioselectivity in entry 1 is due to the desiccation potential of only one of the cyclic aldol adducts. Entry 9, an important step in the synthesis of jatrophones, involves the formation of an eleven-membered ring.
Intramolecular Aldol and Mukaiyama Aldol Reactions
Continued) CH 3
The Robinson Annulation Reaction
Addition Reactions of Imines and Iminium Ions
- The Mannich Reaction
Imines and iminium ions are nitrogen analogs of carbonyl compounds and they undergo nucleophilic additions such as those involved in aldol reactions. Because iminium ions are more reactive than imines, the reactions often proceed under mildly acidic conditions. Addition of enols, enolates, or enolate equivalents of imines or iminium ions provides an important route to amino-ketones.
Synthesis and Utilization of Mannich Bases A. Aminomethylation Using the Mannich Reaction
Amine-Catalyzed Condensation Reactions
These reactions are catalyzed by amines or buffer systems containing an amine and an acid and are referred to as Knoevenagel condensations.211 The reactive electrophile is probably the protonated form of the imine, as it is a more reactive electrophile than the corresponding carbonyl compound.212. First, it allows weak bases such as amines to provide a sufficient concentration of the enolate for reaction. Decarboxylative condensations of this type are sometimes carried out in pyridine, which cannot form an imine intermediate, but which has been shown to catalyze the decarboxylation of arylidenemalonic acids.215 The decarboxylation occurs by concerted decomposition of the adduct of pyridine to the ,-unsaturated diacid. .
Amine-Catalyzed Condensation Reactions of the Knoevenagel Type
Acylation of Carbon Nucleophiles
- Claisen and Dieckmann Condensation Reactions
- Acylation of Enolates and Other Carbon Nucleophiles
In practice, the alkoxide used as base should be the same as the alcohol portion of the ester to avoid product mixtures due to ester interchange. Sodium hydride with a small amount of alcohol is often used as a base for ester condensation. The alkoxide is formed by reaction of the alcohol that is formed as the reaction proceeds.
Acylation of Nucleophilic Carbon by Esters
Continued)
Acylation of Ester Enolates with Acyl Halides, Anhydrides, and Imidazolides
Both diethyl malonate and ethyl acetoacetate can be acylated by acyl chlorides using magnesium chloride and pyridine or triethylamine.223. The products of these reactions are dicarbonyl compounds, which are rather acidic and can be alkylated according to the procedures described in Section 1.2. Alkyl cyanoformate can be used as the acylation reagent under conditions where a ketone enolate is formed under kinetic control.227.
Acylation of Ketones by Esters
Olefination Reactions of Stabilized Carbon Nucleophiles
- The Wittig and Related Reactions of Phosphorus-Stabilized Carbon Nucleophiles
The reaction of a phosphonium ylide with an aldehyde or ketone replaces the carbonyl bond with a carbon-carbon double bond. The stereoselectivity of the Wittig reaction is believed to be the result of steric effects that develop as the ylide and carbonyl compound approach each other. The stereoselectivity depends strongly on both the structure of the ylide and the reaction conditions.
The Wittig Reaction
Continued)
Under these conditions the reaction can be carried out at high temperatures.243 Entries 10 and 11 illustrate this procedure. 2-(1,3-Dioxolanyl)methylylides can be used for the introduction of unsaturated aldehydes (see Entry 15, Scheme 2.17). The reaction can be used to prepare extended conjugated systems, such as crocetin dimethyl ester, which has seven conjugated double bonds.
Carbonyl Olefination Using Phosphonate Carbanions
Continued)
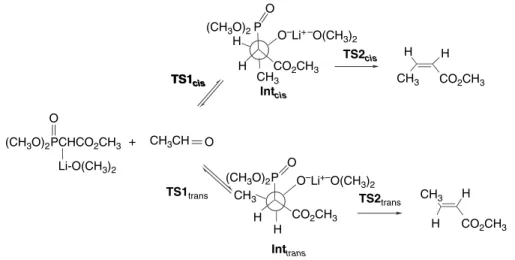
Visual models, additional information, and exercises on the Wadsworth-Emmons reaction can be found in the Digital Resource available at: Springer.com/carey-sundberg. Another computational study included a solvation model.267 Solvation strongly stabilized the oxyanion adduct, suggesting that its formation may be rate- and product-determining under certain conditions. According to the energy profile, its formation is irreversible in solution and therefore determines the stereochemistry of the product.