This work aims to better understand the mechanisms involved in the formation of nanoparticles by laser ablation and by arcing. Subsequently, attention is paid to the role of the background environment and its role in the formation of nanoparticles.
Laser ablation
Spark discharge
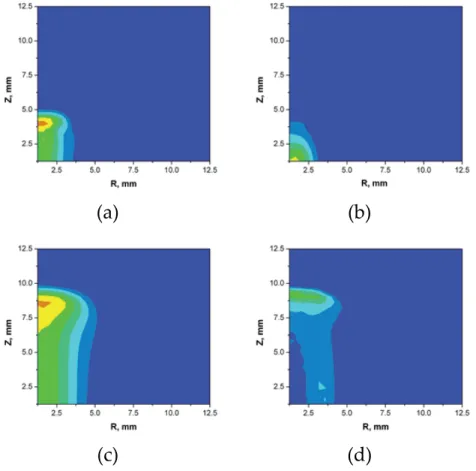
When the polarity is reversed, a crater is formed on the surface of one of the electrodes due to evaporation and erosion. Once the amount of ejected material is calculated, the plasma dynamics is modeled using the Navier-Stokes equations [17].
Spark discharge vs nanosecond laser ablation
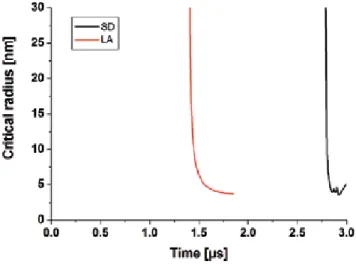
The difference in time delays corresponds to the transition of the system to a supersaturated state. Usually, at the beginning of the diffusion expansion stage, the condition ln(S)=ln(c/ceq)=8πa2σ/3kT ≥1 applies, so that this mechanism dominates the formation of nanoparticles.
Conclusions
The results obtained show that further increase in the number of pulses affects particle size distribution only slightly. In this chapter, recent advances in the modeling of laser ablation and spark discharges are summarized.
Acknowledgements
For example, this process occurs in liquids, especially in the absence of surface passivation by additional chemicals. First, the nanoparticle generation mechanisms are investigated for femtosecond laser interactions in the presence of a background environment.
Author details
Then, conditions are formulated for catastrophic nucleation to become the main mechanism of nanoparticle formation due to thermalization and collisions between the species in the presence of a background environment. This result can be attributed to the fact that while higher vaporization rates are typical for nanosecond laser ablation, a mixture of vapor and background gas determines the supersaturation in the case of sparking.
Fundamentals of Medicinal Application of Titanium Dioxide Nanoparticles
- Introduction
- General mechanism of photocatalysis of TiO 2 NP
- Sterilization effect by TiO 2
- Photodynamic therapy
- Photocatalytic DNA damage by TiO 2 NPs
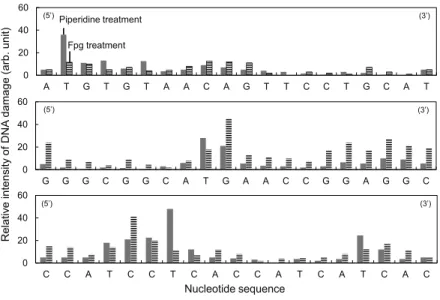
- Isolated DNA damage photocatalyzed by TiO 2 NPs and its sequence specificity Photo-irradiated TiO 2 NPs catalyze DNA damage in the presence of copper(II) ion [31]
- Mechanism of DNA damage photocatalyzed by TiO 2 NPs
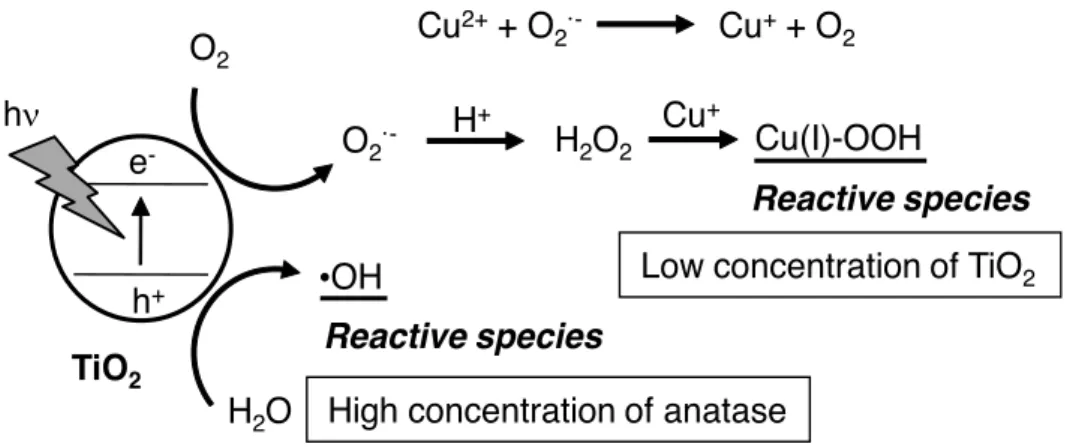
- Secondary production of reactive oxygen species from photocatalyzed materials by TiO 2 NPs
- Singlet oxygen formation through photocatalytic reaction of TiO 2 NPs
Cellular DNA damage photocatalyzed by TiO2 NPs was demonstrated experimentally with cancer cells [18,19,21]. It has been reported that DNA can be a target biomolecule for the photocatalytic reaction of TiO2 NPs [26-30].
Structures
Recent research scenario
- Synthesis procedure
- Characterization
- Morphological investigations
- Structural analysis
- Optical and magnetic properties of Fe 0.5 Gd 0.5 (MoO 4 ) 1.5 :Eu 3+ structures
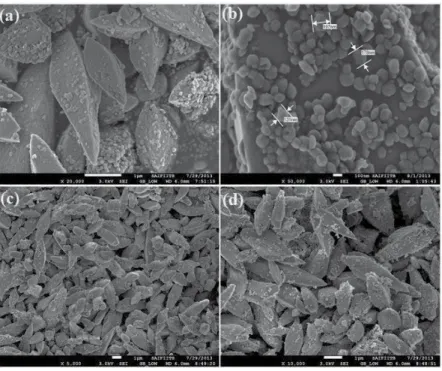
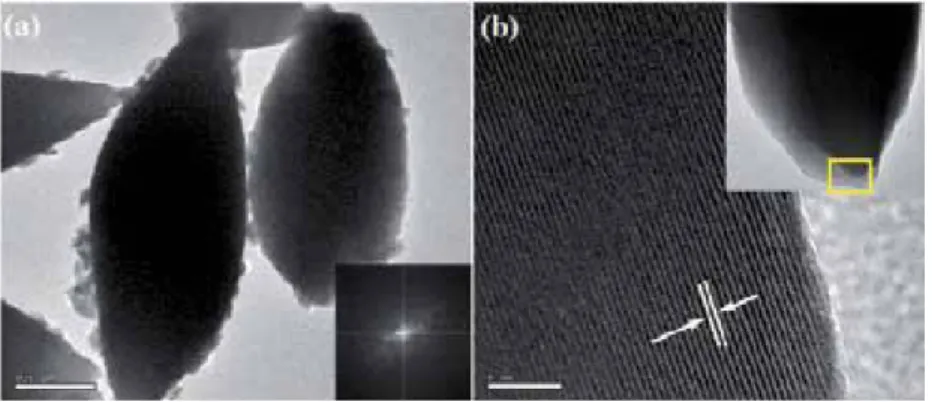
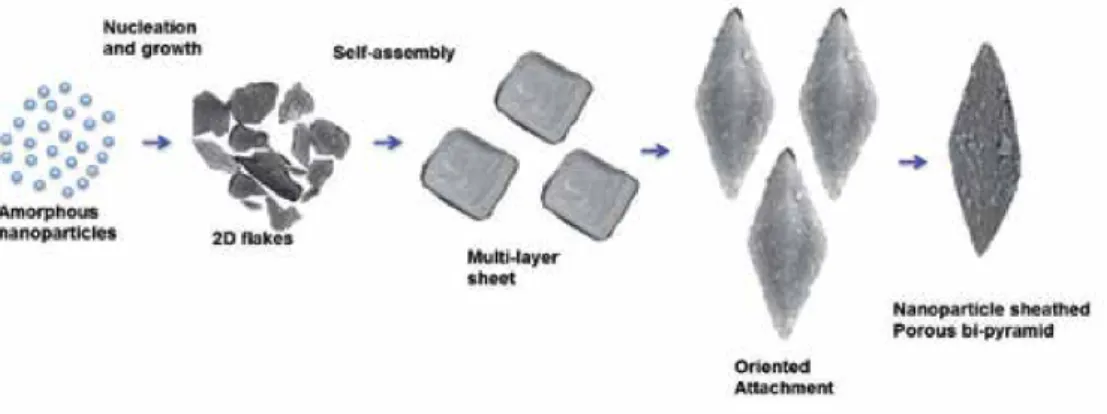
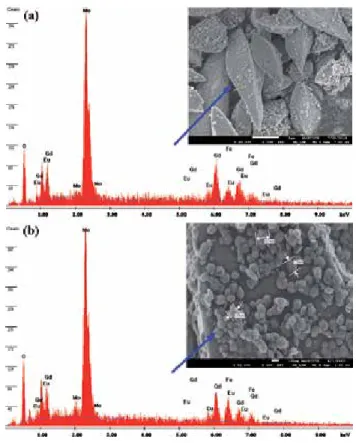
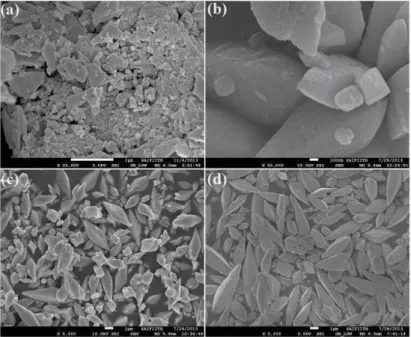
Room temperature magnetic properties of the samples were analyzed using (Lake Shore 7307 model) vibrating sample magnetometer (VSM). Figure (1–3) shows the low, high magnification FESEM image of the Fea0.5Gd0.5(MoO4)1.5:Eu3+ samples synthesized by hydrothermal route at different time inter‐. Due to their similar ionic radius and valence state, the Eu3+ ion successfully replaces the Gd3+ ion and does not change the lattice site of the host Fe0.5Gd0.5(MoO4)1.5:Eu3+ and the crystal phase is very pure.
Due to their similar ionic radius and valence state, the Eu3+ ion successfully replaces the Gd3+ ion and does not change the lattice site of the Fe0.5Gd0.5(MoO4)1.5:Eu3+ host.
Conclusion
Room-temperature emission spectra of Eu3+-doped Fe0.5Gd0.5(MoO4)1.5 synthesized by PVP-assisted hydro-. However, the presence of magnetic iron (Fe3+) in the Fe0.5Gd0.5(MoO4)1.5 tetragonal crystal structure is taken into account. From Figure 8, it is clear that a straight line crosses the origin, indicating that the phosphor Fe0.5Gd0.5(MoO4)1.5:Eu3+ exhibits a paramagnetic behavior due to the existence.
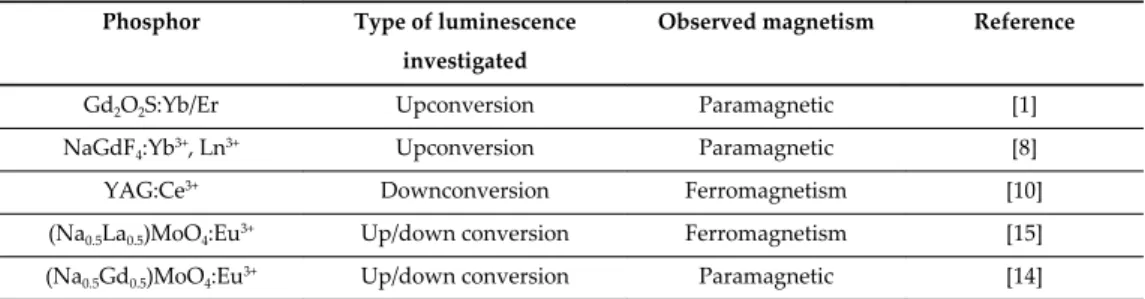
Effect of surfactants on morphology, conversion emission and magnetic properties of β - NaGdF4:Yb3+, Ln3+ (Ln = Er, Tm, Ho).
Effect of Argon Carrier Gas Flux on TiO 2 Nanostructures
- Semiconducting nanowires
- Titanium dioxide (TiO 2 )
- VLS mechanism of nanowire growth
- Experimental details
- Results and discussion
- SEM observations
- Results and discussion 1. SEM observations
- XRD studies
- Optical properties
- Growth mechanism
- Microstructure characterization (theoretical background)
Compared to isotropic nanoparticles and two-dimensional thin films, one-dimensional nanostructures such as nanofibers and nanowires are much more anisotropic, i.e., the length-to-diameter ratio can reach very high values. Analysis of the growth of silicon whiskers (hairs) from the vapor phase using gold catalyst particles leads to the postulation of the vapor-liquid-solid (VLS) growth mechanism. The morphological characteristics of the samples under different carrier gas flows are determined by SEM images.
In the mechanism, the role of the impurity (catalyst) is to form a liquid alloy droplet with eutectoid temperature.
FWHM )
- Microstructure study in TiO 2 nanowires
- The Williamson–Hall integral breadth method
- Dilatation
- Dislocation density
- Texture coefficient
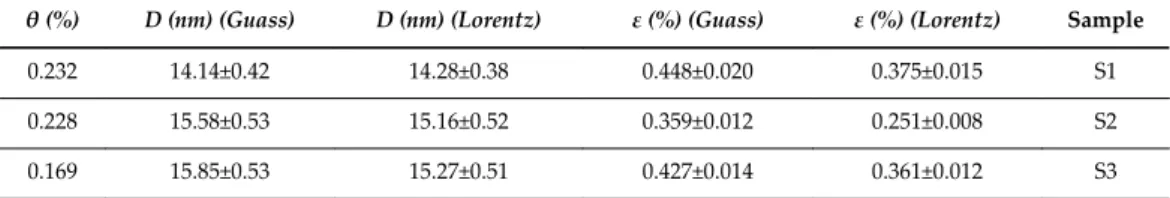
16, the behavior of the dislocation density is similar to the microstrain, i.e., the value of the dislocation density decreases from sample S1 to S2, 4 and then increases from S2 to S3, since the dislocation density and microstrain are related to the displacement . The band gap energy of TiO2 nanowires is increased due to quantum confinement compared to bulk confinement. Wei, Synthesis of Sub-10 Nm TiO2 Nanowires For The Application of Dye-Sensitized Solar Cells, Func.
Liu, et al., Large-scale synthesis of transition metal-doped TiO2 nanowires with controllable overpotential, American Chemical Society.
Noble Metal Nanoparticles Prepared by Metal
Sputtering into Glycerol and their Grafting to Polymer Surface
Nanoparticle synthesis
One of the most common syntheses of precious metal nanoparticles are those developed by Brust–. The authors showed that the formation of AgNPs crucially depends on the applied voltage and the specific surface coordination ability of the used oil (castor oil, rapeseed oil and capron oil). However, as can be seen from the work [52], especially in the case of ILs, other parameters, such as the composition and coordination ability of the liquid used, also play an important role.
In addition, one can easily control the size of emerging particles by the temperature of the capture medium (glycerol).
Gold, silver, platinum, and palladium nanoparticles physically deposited into glycerol
The agglomeration of particles can be seen, which is consistent with UV-Vis analysis. UV-Vis absorption spectra measured on Au and AgNP solutions immediately after deposition (solid lines) and after 3 months of storage at ambient conditions (dashed lines) are introduced in Figure 2 and discussed in detail in [31]. UV-Vis absorption spectra of Au and Ag aqueous nanoparticle solutions together with the photographs of corresponding NP solutions.
UV-Vis absorption spectra of Ag (AgNP4-6) and Au (AuNP4-6, AuNP9-12) aqueous solutions of different nano.
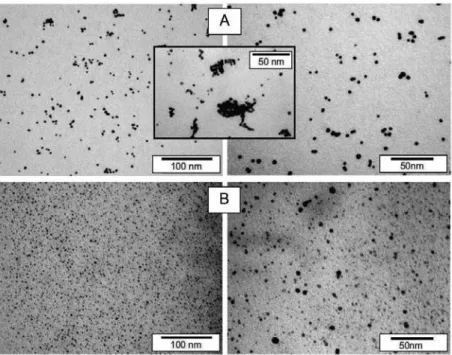
Figure 5
In general, two main aspects determine whether the growth of NPs during metal sputtering into liquids will take place rather than thin film formation on the liquid surface [52]. Apparent aggregation of particles is due to the preparation method of samples for TEM (HRTEM) analysis. Observed discrepancy in particle size is likely due to different sputtering yield of both metals (Pt ~ 1.27, Pd which is consistent with the hypothesized growth mechanism causes significantly slower growth of Pt particles (lower concentration gradient).
Observed particle size discrepancy is likely due to different sputter yield of both metals (Pt ~ 1.27, Pd, which is consistent with the assumed growth mechanism, causes significantly slower growth of Pt particles (lower concentration gradient).
HRTEM
Antibacterial effects of noble metal nanoparticles
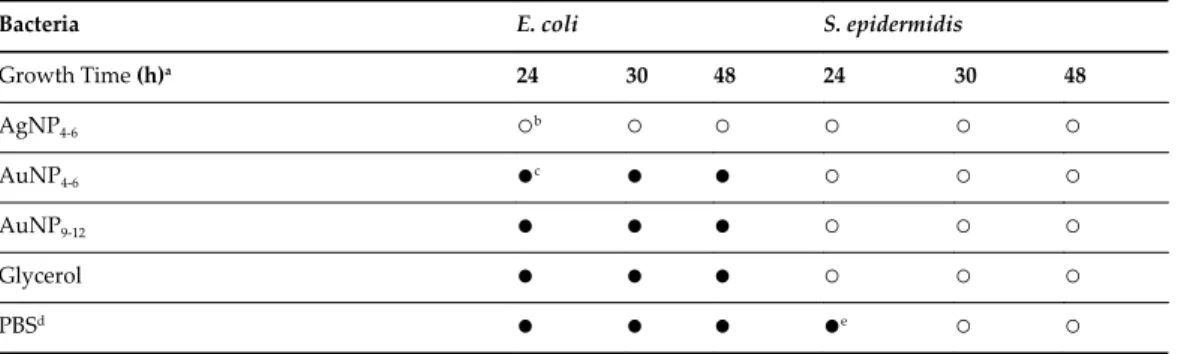
The dependence curve in the case of both Pt and PdNPs indicates the presence of zero-valent metals in the solution [91]. A slight shift of the curves to lower absorbances at red region of visible spectrum (dashed lines) refers to mild agglomeration of individual particles in the solution after 3 months of storage. Inhibitory effect of silver and gold nanoparticles against Gram-negative (E. coli) and Gram-positive (S. epidermidis) bacteria (time length of bacterial growth on LB plates after inoculation, prohibition empty circle indicates inhibitory effect, approx. full circle indicates positive growth , dphysiological saline, a half full circle indicates 50% growth) [58].
Thus, it is very likely that AuNP size also plays an important role in antimicrobial activity.
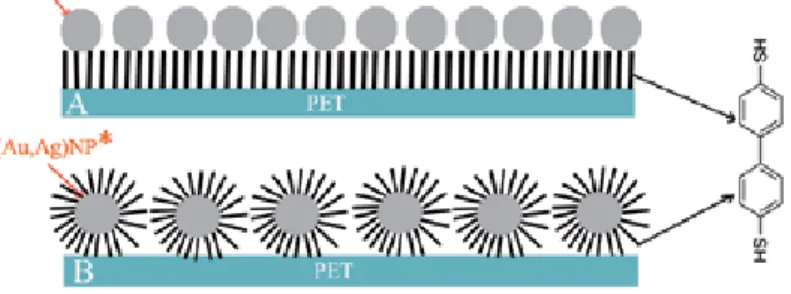
Grafting of noble metal nanoparticles to plasma-activated polymer carriers
At a higher HRTEM resolution (Figure 11C,E), the AuNPs can be seen to have the shape of decahedral particles. It is clear that the behavior of pristine AgNPs (AgNP in Figure 11F) and AgNPs modified with BPD (AgNP* in Figure 11G) is significantly altered. It is clear that the behavior of pristine AgNPs (AgNP in Figure 11F) and AgNPs modified with BPD (AgNP* in Figure 11G) has changed significantly.
From Figure 12 it is clear that the absorption maximum of AuNP* is shifted to longer wavelengths.
Conclusions
Size-controlled synthesis of nearly monodisperse gold nanoparticles in the 1-4 nm range using polymeric stabilizers. UV-Vis and NMR study of gold nanoparticle formation by citrate reduction: Observation of gold and citrate aggregates. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum Size-Related Properties, and Applications in Biology, Catalysis, and Nanotechnology.
Preparation of polymer-coated gold nanoparticles by surface-limited living radical polymerization at room temperature.
The Development of Smart, Multi-Responsive Core@Shell Composite Nanoparticles
Types of Core@Shell nanoparticles
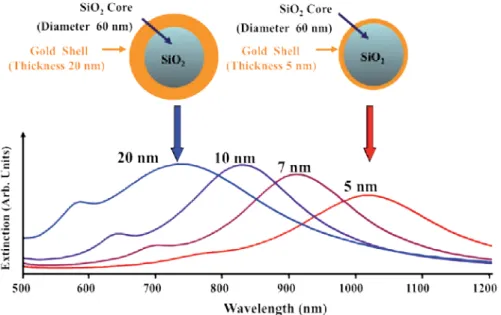
Due to the unique qualities of gold, silica-based core@shell NPs with gold as the shell material became the focus of efforts to produce the first of these structures in the late 1990s. The characteristic surface plasmon peak of the core of these NPs is ∼528 nm before the silica coating is added to the gold cores. Depending on the changes in the thickness of the silica shell, the position of the Bragg diffraction peak varied.
Examples of the types of shapes that can be made (cubic, cuboctahedral and octahedral) are shown in Figure 5 [113].
Smart, multi-responsive Core@Shell nanoparticles
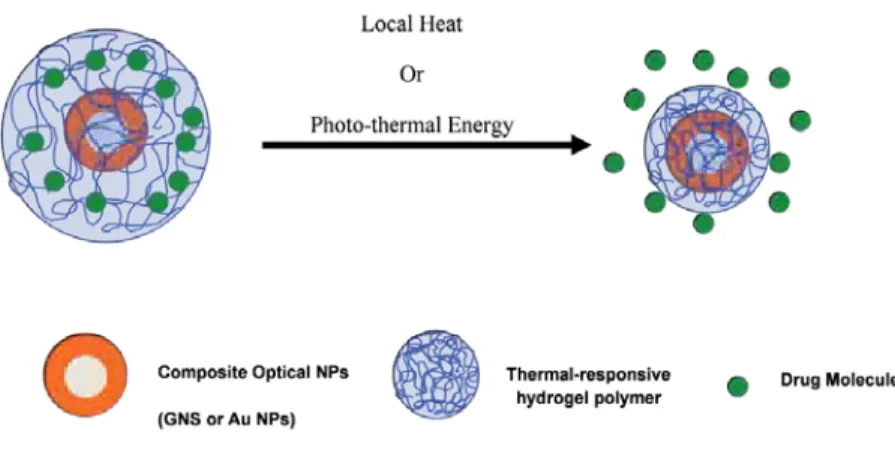
Since specific light frequencies can be used to generate heat on the surface of such light-responsive GNSs, the combination of a well-tuned nanoscale for specific light absorption and a thermally responsive hydrogel offers the potential for drug delivery. remotely controlled. a smart, multi-responsive core@shell nanoparticle system. Furthermore, core@shell composite particles that respond to magnetic stimuli can also be used to perform useful tasks in controlled drug delivery [77, 78], bio-separation [120], chemical catalysis, and electronics [1 , 3]. These core@shell composite particles that respond to both a magnet and other external stimuli typically consist of a magnetic core enclosed in a stimuli-responsive hydrogel copolymer layer that responds rapidly to temperature changes slightly above of the body.
Core@shell composite particles that respond to optical, magnetic, and other external stimuli (eg, temperature, ionic strength, and pH) can be used to perform more useful tasks in research.
Synthesis of smart multi-responsive Core@Shell nanoparticles
To overcome this obstacle, our research group has explored the growth of a stimuli-responsive hydrogel polymer layer on gold nanogods that can release a model drug after the collapse of the hydrogel matrix. For many hydrogel applications, heat is used to initiate the collapse of the polymer hydrogel. By integrating the application of their physical and chemical properties, these core@shell magnetic materials can become multifunctional devices that enable a variety of advanced applications that cannot be realized by simple magnetic NPs alone.
Recent examples of the application of magnetic NPs in research have demonstrated their utility due to their ability to generate heat under an external oscillating magnetic field or to be remotely manipulated, allowing their use as an anti-tumor treatment, cell tracking label or drug delivery vehicle [123- 125].
Long term research objectives
Fine-tuning the LSPR response of gold nanorod-polyaniline core-shell nanoparticles with high photothermal efficiency for cancer cell ablation. Facile synthesis of functionalized superparamagnetic magnetite/silica/core/shell nanoparticles and their use as magnetically separable high-performance biocatalysts. Shape and size control of cubic, cuboctahedral and octahedral Cu-Cu2O core-shell nanoparticles at the nanometer level on Si(100) z.
Synthesis of Dumbbell-Shaped Au-Ag Core-Shell Nanorods by Seed-Mediated Growth under Alkaline Conditions.