Despite the high density of valuable information, this textbook is a good read and I am fully convinced that it will surely become one of the main reference texts in the field of polymer sciences. We also thank the many interested readers and critics of the German edition who contacted us to point out errors and typos.
Introduction and Basic Concepts
- Polymers: Unique Materials – 2
- Definition of Terminology and Basic Concepts – 4 .1 Fundamentals – 4
- Polymers: Unique Materials
- Definition of Terminology and Basic Concepts
- Fundamentals
- Polyreactions
- Nomenclature of Polymers
- Polymer Architectures
- Linear and Branched Macromolecules
- Isomerism in Polymers
- Copolymers
In the following, a brief overview is given of the basic possibilities for the synthesis of polymers (polyreactions). The following chapters provide a deeper discussion of the properties and synthesis of polymeric materials.
5 The handbook concludes with some environmental aspects of synthetic macromolecules (7 see chapter 21) and a brief presentation of current trends in polymer chemistry (7 see chapter 22).
Polymers in Solution
Chain Models
These considerations lead to an extension of the polymer coil compared to the Gaussian chain. Accounting for a fixed joint angle thus leads to a doubling of the average square end-to-end distance.
Chain Stiffness
It should be noted that the persistence length is a mathematical paraphrase of the stiffness of the polymer chain. This reiterates that the persistence length and the Kuhn length are a measure of the stiffness of the polymer chains.
Entropy Elasticity
The elasticity of a polymer chain, which in macroscopic terms corresponds to the resistance of a polymer material to deformation, depends on the entropy of the chain and is thus proportional to the average end-to-end distance between chain ends.
Thermodynamics of Polymer Solutions
- Ideal and Real Solutions
- Solutions of Lower Molecular Substances
- Polymer Solutions
The shapes of the curves of the free enthalpy of mixing as a function of φ2 for different values of χ are shown in However, if some miscibility remains, it is due to the contribution of the entropy of the system.
Polymer Analysis: Molar Mass Determination
This chapter focuses on how the molar mass of polymers and their molar mass distribution can be mathematically described and measured experimentally. Experimental methods for determining molar mass can be divided into two basic categories: those referred to as “absolute methods,” the results of which can be directly converted to a molar mass, and a second group of methods, the “relative methods,” from which the results should be calibrated with samples of known molar mass to derive the molar mass of the unknown sample.
Definition of Molar Mass Parameters
It is given by the product of the number of molecules with a molar mass Mi, ni, and their individual molar mass Mi. The mass of all molecules with the molar mass Mi is given by the product of ni and their individual mass Mi.
Absolute Methods
- End Group Analysis
- Colligative Properties
- Membrane Osmometry
- Vapor Pressure Osmometry
- Ultracentrifuge
- Analysis of Sedimentation Velocity
- Measurement at Thermodynamic Equilibrium
- Sedimentation Equilibrium in a Density Gradient
- Light Scattering
- Static Light Scattering
- Dynamic Light Scattering
- MALDI-TOF-MS
- General
- Basics
If the densities of the particle and the surrounding liquid are known, the molar mass can be calculated. The discharge in relation to the concentration of the dissolved substance is given by dG. Since the intensity is proportional to the square of the amplitude (I= E2), the second approach makes the approximation that qR is extremely small so that the shape factor P(q) can be given by.
Relative Methods
- Viscometry
- Size Exclusion Chromatography (SEC)
The molar mass of individual chains is separated by the mass of one styrene unit. A major advantage of viscometry lies in the large range of molar masses that can be determined. The range of molar mass that can be measured depends on the exclusion limits of the separation column.
Polymers in Solid State
- Phase Transitions in Polymeric Solids – 95
- Methods for the Determination of T G and T m – 97 .1 Static Procedures – 97
- Phase Transitions in Polymeric Solids
- Methods for the Determination of T G and T m
- Static Procedures
- Dynamic Procedures
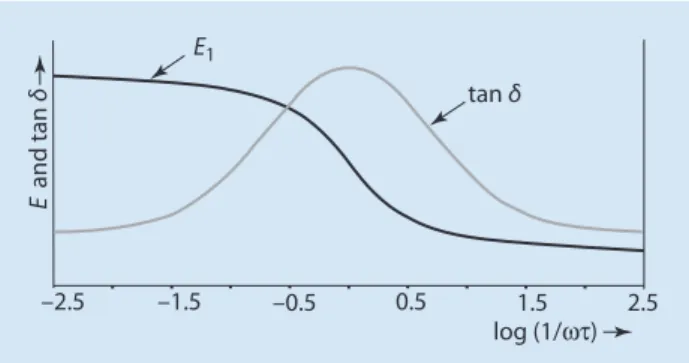
This means that the applied stress σ (force per unit area) is proportional to the elongation of the test ε. Examples are dilatometry (7 see Sect. 5.3) and refractive index measurement, where discontinuities in volume or refractive index curves can be observed as a function of temperature at the glass transition temperature. At low temperatures, the mobility of the material is not sufficient to respond to strain by changing the helix conformation.
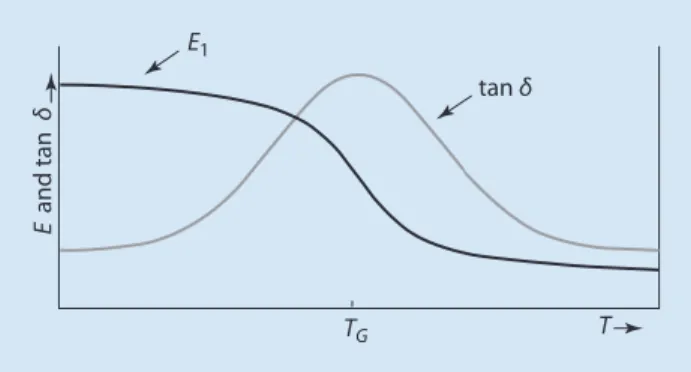
In principle, DETA can be used in the same way as DMTA; however, it can only be applied to polymers with dipolar groups. Thus, in combination with DMTA, DETA allows conclusions about whether dipolar groups are involved in a particular transition.
Partially Crystalline Polymers
Factors that Influence Melting Temperature
- Influence of the Conditions for Crystallization
- Influence of Chain Flexibility
- Influence of Chain Symmetry
- Influence of Interactions Between the Chains
- Influence of Tacticity
- Influence of Branching
- Influence of Molar Mass
- Comonomers
The resulting disruption in the organization of the crystals leads to a reduction in the melting temperature. A rule of thumb is that the glass transition temperature (in Kelvin) is about 0.7 times the melting temperature. The resulting disruption of the crystalline order leads to a drop in both the melting temperature and the module.
Morphology of Partially Crystalline Polymers
The differences can be mainly attributed to molar mass, the degree of branching, tacticity and the thermal history of the material (cooling rate, tempering). Therefore, it is false that any one of the listed values is more or less correct than any of the others. The question of how the polymer chains fold at the ends of the lamellae has not been conclusively resolved.
Crystallization Kinetics
This equation describes the weight fraction of the non-crystalline melt at time t relative to the original mass of the melt at time t0. From the value of the Avrami exponent, n, it is possible to predict what kind of superstructures form in the melt. Natta G, Pasquon I, Zambelli A, Gatti G (1964) Dependence of the melting point of isotactic polypropylenes on their molecular weight and the degree of stereospecificity of different catalytic systems.
Amorphous Polymers
Dependence of the Mechanical Properties of Amorphous Polymers on Temperature – 120
Glass Transition – 122
Factors that Influence the Glass Transition Temperature – 122
Dependence of the Mechanical Properties of Amorphous Polymers on Temperature
At this temperature, the material can respond better to an external force; the polymer chains can rearrange and the material is less brittle. However, this changes if mechanical force is applied to the material over a longer period of time. Over temperature range III, the material is, theoretically, an extremely viscous melt, called the rubber-elastic state.
Amorphous State
The latter, at the molecular level, involves polymer chains that react to an applied force by assuming a different conformation. By moving relative to each other, the polymer chains can rearrange into an energetically more favorable conformation if the applied stress is applied for a longer time or temperatures are higher. An increase in temperature leads to thermal expansion of the material, the free volume increases, and the polymer chains become more mobile; viscosity decreases and melts flow more easily.
Glass Transition
This is the case both above and below the TG, and as a consequence the density of the amorphous material is lower than the density of the corresponding crystal.
Factors that Influence the Glass Transition Temperature
- Chain Flexibility
- Steric Effects/Substituents
- Tacticity, Branching/Cross-Linking, and Molar Mass
- Plasticizers
Thus, the glass transition temperature rises asymptotically to a limiting temperature valid for very high (infinite) molar mass. The effect of adding a plasticizer on the glass transition temperature can be estimated using TG, W Freezing point or glass transition temperature of the plasticizer TG Glass transition temperature of the plasticized polymer.
Rheological Behavior of Polymer Melts
- Newtonian Fluid
- Non-Newtonian Fluids
- Process of Reptation
The entanglement of the polymer chains is the main reason for the high viscosity of polymer melts. The relaxation time is thus proportional to the square of the chain length and indirectly proportional to the diffusion coefficient Dt of the polymer chain in the tube. Due to the dependence of the diffusion coefficient on chain length, if effective entanglements exist, the relaxation time of the polymer chain is proportional to the chain length cubed, consistent with the gradient of the line in.
Viscoelasticity
- Influence of Time on the Mechanical Behavior
- The Maxwell Approach
- Voigt–Kelvin Model
- The Burgers Model
Alternatively, during a prolonged application of force, a reorientation (or at least a partial one) of the polymer chains relative to each other takes place. This results in a movement of the polymer chains relative to each other—the material begins to flow. 5 Time-delayed reaction of the material (phase shift) if an oscillating force is applied. The latter is the basis for the dynamic-mechanical-thermal analysis (DMTA) which is presented in 7 Ch.
Polymers as Materials
Cross-Linked Materials – 154
Depending on the size of the production run, polymer articles can have relatively low manufacturing costs. Last but not least, the relatively low temperatures at which synthetic materials are usually processed lead to energy and consequently cost reduction during processing. As a result, many polymer researchers spend their time developing macromolecules that can be tailored to specific requirements.
Fracture Behavior
The material is characterized by a high Young's modulus, i.e. the material is very stiff and a large force is needed to stretch it. The strength of the material, the stress at which the material breaks, tends to decrease. It is important to note that the maximum strength of the material, i.e. the maximum energy required for fracture, is reached at an average temperature T4.
- Mechanical Characteristics
- Optical Characteristics
- Materials for Lightweight Construction
- High-Temperature Materials
- Cross-Linked Materials
- Structure and Application of Networks
- Mechanical Characteristics of Networks
- Network Synthesis
- Typical Cross-Linking Reactions
- Polymer Additives
- Technological Requirements on Polymer Additives
- Function of Selected Additives
Cross-linking is one of the most important means of modifying the material properties of a polymer. Both crystalline and amorphous polymers can be crosslinked, whereby crosslinking to a first approximation does not change the morphology of the material. The mechanical properties of the polymers are affected by plasticizers or reinforcing fillers (glass fiber, 7 ch. 17).
7.4.3 “White Carbon Black”
An unavoidable side effect is a deterioration of the electrical insulating effect because additives are usually more polar than the polymer. One of the most effective additives against the negative effects of light is soot. In this way, the danger of electrostatic charge on PVC floors, for example, can be reduced.
Step-Growth Polymerization
Differences Between Step-Growth and Chain-Growth Polymerizations – 165
Molar Mass, Degree of Polymerization, and Molar Mass Distribution – 167
Polymerizations – 175
Differences Between Step-Growth and Chain-Growth Polymerizations
A typical example of step growth polymerization is the synthesis of nylon 6.6 from 1,6-hexamylenediamine and adipic acid. In contrast to chain growth polymerization, high molar mass polymers take a long time to form. Given the criteria described here, polymerization reactions can be unambiguously classified into chain growth and step growth polymerization.
Molar Mass, Degree of Polymerization, and Molar Mass Distribution
- Degree of Polymerization and Molar Mass of Step-Growth Polymerizations
In a typical condensation reaction, for example an esterification of a dicarboxylic acid with a diol, the molar mass of the polymer decreases with (2n − 1) · H2O (and with AB monomers such as a 6-hydroxycarboxylic acid with (n) − 1) · H2O). If the concentration of the reactive groups is not stoichiometric, the degree of polymerization can be determined from the following considerations. 1 The concentration of the functional groups is the same as that of the existing molecules; the former is more easily accessible experimentally.
Molar Mass Distribution of Step-Growth Polymerization
The conversion of an AB monomer to an [AB]P polymer involves (P − 1) polymerization steps (for example, esterifications). The probability of forming a polymer molecule, with exactly P repeating units, requires not only (P − 1) reactions of the functional group A, but also that one A group does not react. This PDI is the same as the PDI for radical polymerizations where termination occurs via disproportionation (7Sect. 9.3).
Linear, Branching, and Crosslinking Step-Growth Polymerizations
- Monomers with Two Different Functional Groups
- Reaction of Two Different Monomers Each Having Two Identical Functional Groups
- Polymers from A x B-Monomers (x ≥ 2)
- Cross-Linking
The problem of the stoichiometry of the reactants 1,6-hexamylenediamine (AA) and adipic acid (BB) can be elegantly avoided by pre-mixing the ingredients and converting them into a so-called AH salt that can be isolated in its solid state. Here pB is the probability that B has reacted and P is the probability of the reaction of A with B. The conversion at the gel point as a function of the functionality of the reaction mixture is given.
Kinetics of Step-Growth Polymerization
The rate of esterification thus depends linearly on the alcohol, carboxylic acid and proton concentrations. Because external catalysis causes the average degree of polymerization to increase linearly with time, external catalysis is preferable to self-catalysis, where the degree of polymerization is proportional to the square root of time.
Typical Polycondensates
- Polyester
- Polycarbonates (PC)
- Polyestercarbonate (PEC)
- Aliphatic Polyamides (PA)
- Partially Aromatic and Aromatic Polyamides and Polyimides
- Polymers of Isocyanates
- Polysiloxanes
The reaction of bisphenol A with diphenyl carbonate can be carried out as a melt condensation; the crucial reaction here is a transesterification. In contrast, the main chain in PA 6 is less symmetric—the mirror plane present in PA 6.6 is missing. Typical reactions of isocyanates which can be used for a stepwise synthesis of polymers are summarized in.