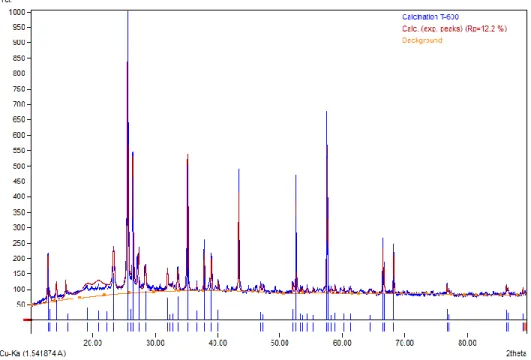
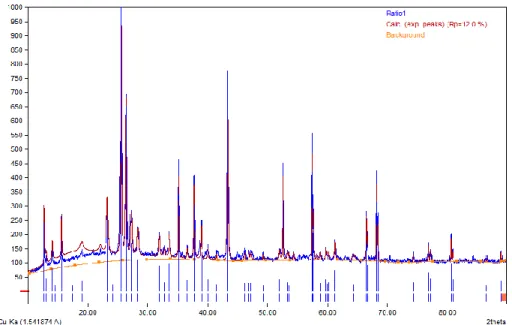
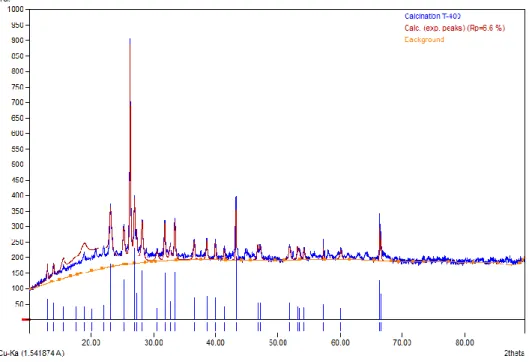
The thermogravimetry analyzer (TGA) method was used to determine the regeneration temperature of the nanocatalyst. These three processes show that the regeneration cycle of the nanocatalyst is complete at the selected temperature.
Research background
Using catalytic cracking process, including thermal cracking and two-step processes, this study investigates the optimum conditions for the production of biogasoline from waste cooking oils. The investigation will extend to catalytic cracking activity to determine the effects of reaction temperature, weight hourly space velocity (WHSV) and catalyst weight.
Problem statement
Transesterification is used as an advanced pretreatment step to remove residual impurities from the raw material. The transesterification is used as a progressive pretreatment step simply because waste cooking oil cannot be converted into biogasoline through transesterification alone.
Importance of the topic
It is suitable to generate biodiesel and bio-gasoline from waste cooking oil and biogas derived from wastewater. Knothe [9] stated that several studies investigate the production of a variety of biofuels (bio-gasoline in the context of this study) using edible and non-edible raw materials such as vegetable oil, palm oil and waste cooking oil.
Aim & Objectives
Aim
The end use of biogas has a decisive influence on its purification - the purification of biogas, regardless of its end use, reduces the concentration of hydrogen sulphide due to its nuisance. 131], the regeneration cycle of the catalyst increases due to combustion of coke on its surface.
The Objectives of this work include
Research contributions
The second section of the discussion focuses on the pre- and post-treatment of waste cooking oil. The presence of the catalyst accelerates the reaction rate and selectivity of the yields. Moreover, these side reactions lead to the entrapment of the produced biodiesel in the soap.
The removal of the organic material from the surface of the catalyst was reacted by combustion with air. The presence of long-chain fatty acids in WCO increases the heating value of the oil. The effect of the weight ratio of the catalyst was investigated at a constant temperature, reaction time (60 minutes) and gas flow rate.
It determines the correct production route (method or technique) and production costs of biogasoline.
Overview of thesis
Introduction
Products such as coal, gas and all crude oils fall into the fossil fuel category. Taking into account that the entire world depends on fossil fuels as a source of energy, the disproportionality in the distribution of fossil fuels results in the entire planet's dependence on rich countries for energy.
Biorefinery concept
Conversion Platforms
The rise in CO2 levels in the atmosphere is directly proportional to the number of fuels used, leading to global warming, acid rain and climate change. The distillation column purifies the products based on the difference in boiling points of the components in the mixture.
Feedstocks
The hybrid conversion platform combines three conversion platforms (biochemical, biological and thermochemical) to produce valuable biomass products. Purification of products is carried out using a distillation column (trays or packed columns) and evaporation.
Classification of biofuel generations
- First-generation biofuel
- Second-generation biofuel
- Third-generation biofuel
- Fourth-generation biofuels
The production of second-generation biofuels saw the light of day to overcome the limitations of the first generation. The feedstock used to produce first-generation biofuels (FGBs) has no difference in properties from that used to produce second-generation biofuels (SGBs).
![Figure 2. 1: Production pathways to liquid fuels from biomass and, for comparison, from fossil fuels [28]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/31.918.129.789.122.467/figure-production-pathways-liquid-fuels-biomass-comparison-fossil.webp)
Review of biofuel products
- Bioethanol
- Biogas
- Biodiesel
- Biogasoline
The method used to produce biogasoline is similar to that used in the oil refining industry. This empirical model explained why a higher flash point in the fuel contains more extended carbon chain linkages.
![Figure 2. 4: General scheme for transesterification of triglycerides [42]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/38.918.162.785.122.332/figure-general-scheme-transesterification-triglycerides.webp)
Biogasoline production processes
- Catalytic cracking
- Hydrocracking
- Thermal cracking
- Two-step process (Hybrid process)
- The catalyst used in the production of bio-gasoline
The conversion of waste cooking oil (WCO) to biogasoline uses the following processes: catalytic cracking, hydrocracking and thermal cracking [71]. Catalytic cracking is a thermochemical conversion process that converts long carbon chain molecules (higher weight petroleum constituents) into shorter carbon chains (lower molecular weight) in the presence of a catalyst. It is simply used as an advanced pre-treatment step of waste cooking oil prior to catalytic cracking.
![Table 2. 9: Biogasoline production from vegetable oil by the catalytic cracking process using zeolite catalyst[ 85]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/50.918.110.811.720.1092/biogasoline-production-vegetable-catalytic-cracking-process-zeolite-catalyst.webp)
Biogasoline optimization
Box-Behnken design optimization method (technique)
There are a number of surface design multivariate methods, to name a few: central composite, Doehlert Matrix (DM) and three-level full factorial design; however, BBD is an effective model regardless of the number of factors considered or calculated. Techniques such as three-level full factorial designs cannot handle the higher number of factors exceeding two.
Optimization model
Therefore, the production of biogasoline by catalytic cracking of waste cooking oil uses a fixed-bed reactor. 121] stated that the design and construction of a fixed-bed reactor is reliable and simple to treat sources such as waste cooking oil. 121, 122] added that the fixed-bed reactor feedstock system is suitable for small-scale projects.
![Figure 3. 1: Schematic set-up [122]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/55.918.255.703.671.953/figure-schematic-set-up.webp)
Introduction
Materials used
Equipment used
Experimental Setup
Fixed bed reactor ................................................................. Error! Bookmark not defined
Introduction
This chapter describes the steps taken in the laboratory to produce biogasoline. There are steps used in making biogasoline by catalytic cracking of waste cooking oil: waste cooking oil pretreatment, catalyst synthesis, thermal cracking, catalyst cracking, combined catalyst cracking and transesterification (two-step process), and catalyst characterization.
Waste cooking oil pre-treatment
The solid particles removal
Three sieves were set as follows and 300 µm, the purpose of sieving the oil was to remove any solid particles in the oil. The sieves were set up on the pulverizer used in mineral processing for crushing. The large aperture sight was placed on top of the setup and the small aperture size was placed at the bottom.
Desalting
Catalyst preparation
Thermal cracking
50 ml of waste cooking oil was poured into the tube for each run and - The reactor was switched on; the reactor temperature was set. The first recipient sits in an ice bath, the second in a regular ice bath, and the third in a dry ice bath throughout the experimental procedure. Since the carrier gas flow rate does not affect the product, the gas pressure and temperature were constant, 50kPa and 25oC.
Catalytic cracking
This conclusion is based on an estimate for conventional gasoline boiling in the range of 30–220 °C (Government of Canada, n.d.). A thermal analysis of the reaction time effect at different temperatures was performed. The organic liquid product was then analyzed using gas chromatography-mass spectrometry (GC-MS).
Two-step process (Hybrid method)
Technical analysis
- X-ray diffraction (XRD)
- Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
- Scanning and Transmission Electron Microscopy (SEM & TEM)
- Gas Chromatography and Mass Spectrometry (GC-MS)
- Volume and Mass measurement: Density
- Viscosity measurement
- Thermogravimetric analysis (TGA)
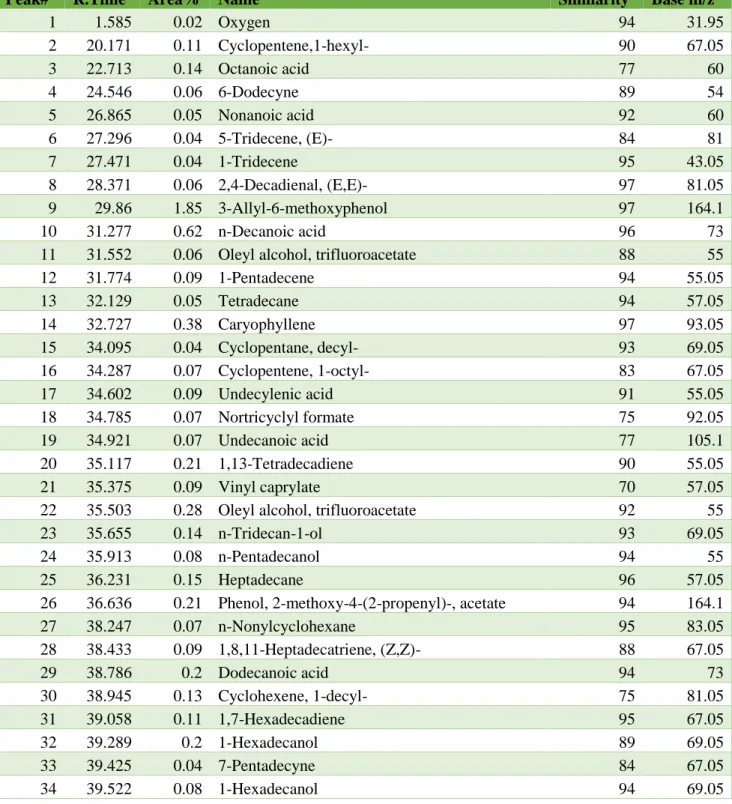
The quality of the image depends on the resolution of the equipment used to analyze the sample. The acceleration voltage is the parameter used in microscopy analysis to determine the speed of the electron. The change in weight of the material under consideration is a function of temperature or time[129].
Introduction
Catalyst characterization
- X-ray diffraction (XRD)
- Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
- Scanning and transmission electron microscopy
- Determination of regeneration temperature
The same study reported that the negative trend observed in the derivative curve is due to the combustion of different coke properties on the catalyst surface [130]. There is a clear indication that the catalyst fired at 700oC cannot be regenerated after use. The regeneration conversion was 100% for the catalyst calcined at 600oC, indicating that it can be completely regenerated and reused.
Waste cooking oil pre-treatment
The composition and purity of the feed play a crucial role in determining the activity, selectivity and life of the catalyst. Therefore, it is essential to ensure a high quality and consistent feed in the biogasoline production process to maximize the performance and efficiency of the catalyst. It is beneficial for the catalyst to remove impurities from waste cooking oil, especially salts.
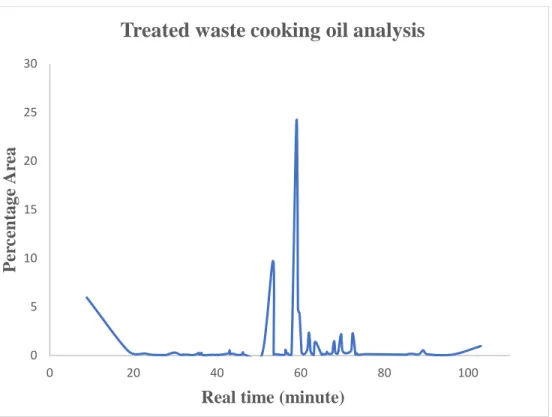
Bio-gasoline product
- Bio-oil percentage yield
- Bio-oil analysis
- Thermal cracking method
- Catalytic cracking method
- Two-step process (Hybrid Method)
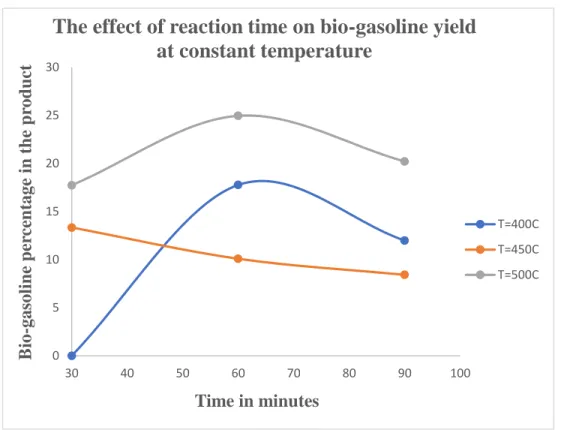
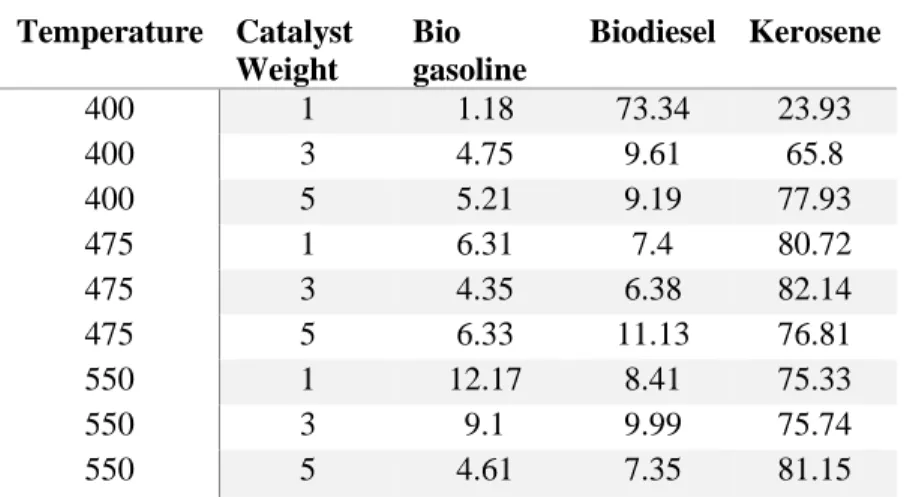
As shown in the following figure, increasing the reaction time does not necessarily lead to an increase in the yield of the biogasoline product. It was found that the percentage of biogasoline in the product increased with increasing catalyst calcination temperature. In the second stage of the two-stage process, the FAME produced in the first stage is subjected to catalytic cracking.
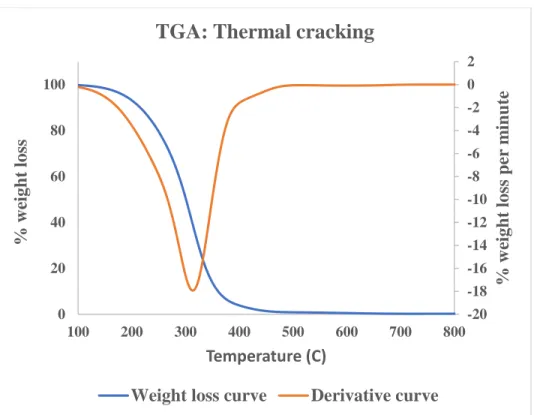
Thermal stability of bio-gasoline
Therefore, the weight loss curve is constant in the early stage of the thermogravimetric analysis before decreasing with increasing temperature. The weight loss curve shows changes at a temperature close to 300oC, while the derivative curve varies between 300-800oC. In their study, the weight loss curve started to change around 350oC, and the derivative curve changed between 350-800oC.
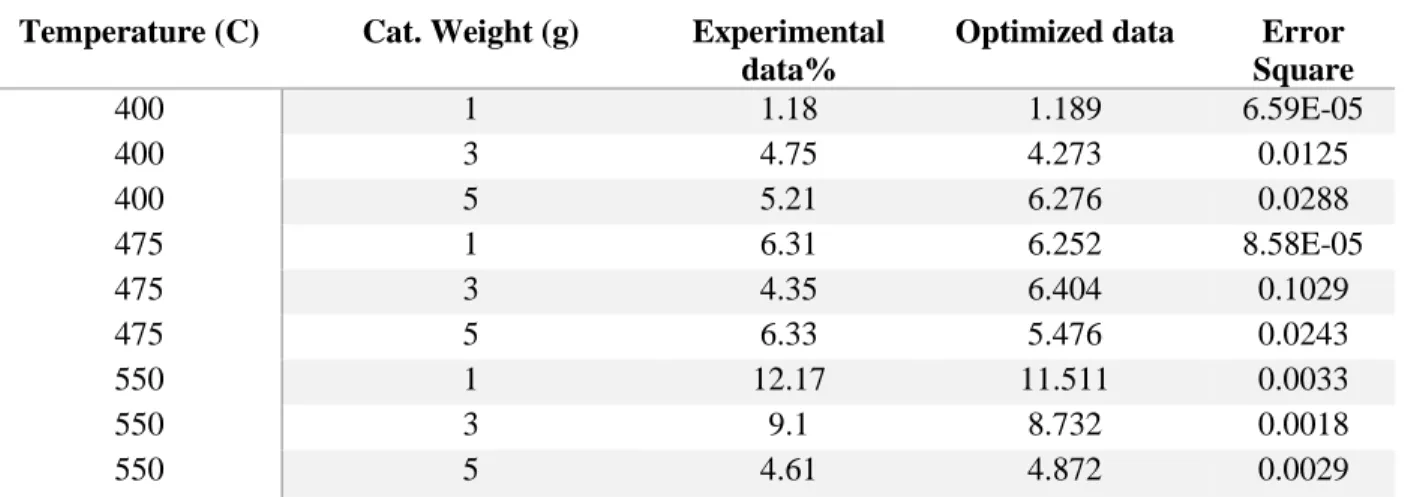
Optimization
Catalytic cracking
Therefore, the assumption that the nitrogen flow rate does not affect the result is correct. The R-Square value is an indication of how well the data fit the model used. In the regression statistics table 5.5, the R-squared value is 0.469, which means that the model can explain about 47% of the variation in the data.
Two steps process (hybrid process)
From the ANOVA table, the significance of the F value explains that the regression of data was good as the significance of the F value is less than the α value (α = 0.05). The significance of F for the two-step process is lower, which explains the observed regression.
Weight hourly space velocity (WHSV)
By-products
A second X-ray diffraction analysis was performed to evaluate the influence of the molar ratio of the components on the catalyst samples. Therefore, the catalyst was calcined at high temperatures ranging from 500oC to 700oC to perform catalytic cracking of waste cooking oil. The catalytic cracking was performed based on the calcination temperature, the weight load of the catalyst and the reaction temperature.
Pilot-scale catalytic thermal cracking of crude palm oil: Effect of Na2CO3 percentage on biofuel quality. Thermal/Catalytic Cracking of Hydrocarbons for Olefin Production: A State-of-the-Art Review I: Review of Thermal Cracking.
Economic assessment of the production of biogasoline ............. Error! Bookmark not defined
Introduction
Colour
Density
Viscosity
Conclusions
The graphic representation indicated that the catalyst calcined at 600oC underwent the regeneration cycle more effectively than the other two catalyst samples. Therefore, the catalyst calcined at 600oC with a smooth regeneration response curve was considered suitable for the production of biogasoline by catalytic cracking using waste cooking oil. This abnormality can be linked to the amount of coke deposited on the catalyst during calcination.
Recommendations
Based on the study of the biogasoline production at the constant reaction temperature (475oC) under variation of the calcination temperature, the biogasoline percentage yield increased with the calcination temperature. Production of biogasoline from waste cooking oil as an environmentally friendly alternative liquid fuel: University of the Witwatersrand, Faculty of Engineering and the Built …; 2017. Bonded cleavage at carboxyl group-glycerol backbone position in thermal cracking of the triglycerides in sunflower oil.
![Figure 2. 2: Simplified depiction of process steps for the production of second-generation fuel ethanol [28]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/32.918.115.801.113.329/figure-simplified-depiction-process-production-second-generation-ethanol.webp)
![Figure 2. 3: Simplified depiction of process steps for thermochemical biofuel production [28]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/33.918.155.760.297.634/figure-simplified-depiction-process-steps-thermochemical-biofuel-production.webp)
![Figure 2. 5: Molecular structure of triglyceride (vegetable oil) molecule consisting of oleic and linoleic acid chains [45]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/40.918.161.757.197.406/figure-molecular-structure-triglyceride-vegetable-molecule-consisting-linoleic.webp)
![Table 2. 11: Biogasoline production from vegetable oil by the catalytic cracking process using modified zeolite catalyst with the addition of metals [85]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10332521.0/52.918.113.811.329.888/biogasoline-production-vegetable-catalytic-cracking-modified-catalyst-addition.webp)