Western blot analysis confirmed that expression of PFF1010c occurs at the gametocyte stage of parasite development. This study confirmed that PFF1010c is only expressed at the gametocyte stage of the malaria parasite.
Malaria as a global health burden
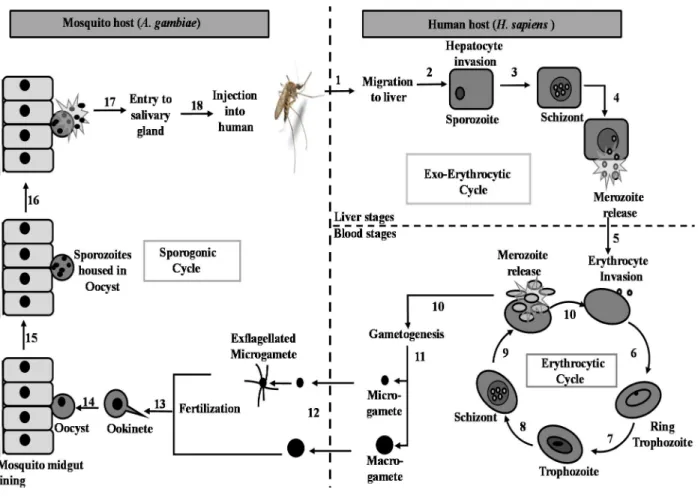
Malaria and life cycle of Plasmodium
The sporozoites released from the mosquito's salivary glands enter the bloodstream during feeding and invade hepatocytes (Figure 1.1; Stage 2). Afterwards, the oocyst ruptures and releases sporozoites, which eventually migrate to the mosquito's salivary glands (Figure 1.1; Stages 14 -18).
Gametocyte stage of malarial parasite
These gametocytes are ingested by female Anopheles mosquitoes during a blood meal (Figure 1.1; stages 11-12; Fujioka and Aikawa, 2002). The resulting ookinete traverses the mosquito gut wall and encysts on the exterior of the gut wall as an oocyst (Figure 1.1; stages 12–13).
Molecular chaperones
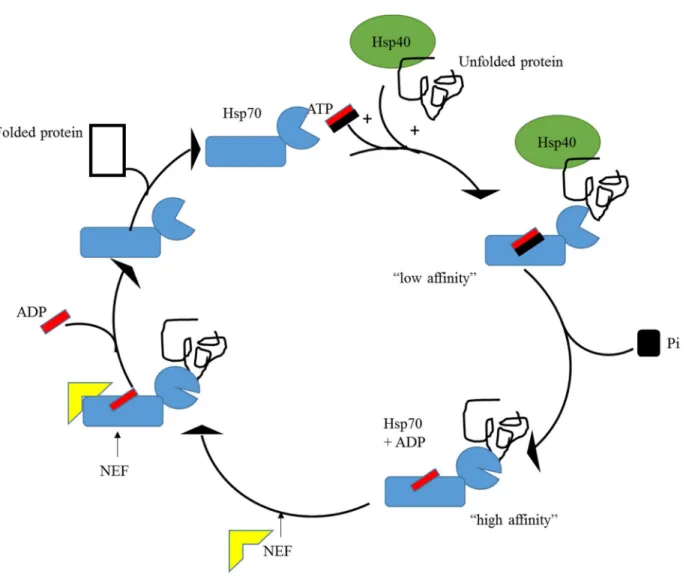
Of the five morphologically distinct gametocyte stages (I to V) (Hawking et al., 1991), only one (stage V) is associated with the blood stage (Okell et al., 2008). Other chaperones act as holdases, and their function is to bind folding intermediates to prevent their aggregation (Hoffmann et al., 2003; Kampinga and Craig, 2010).
Heat shock proteins
- Hsp100 proteins
- Hsp90 proteins
- Hop
- Hsp60 proteins
- Hsp70 proteins
- Hsp40 proteins
- The Hsp70/Hsp40 partnership
- P. falciparum Hsp70 proteins
- P. falciparum Hsp40 proteins
Hsp100, also known as caseinolytic protease (Clp) belongs to the ATPase protein family associated with various cellular activities (AAA) (Gottesman et al., 1997). PfHsp70-x localizes to the PV and is exported to the cytosol of infected red blood cells (iRBC) ( Külzer et al., 2012 ).
Study motivation
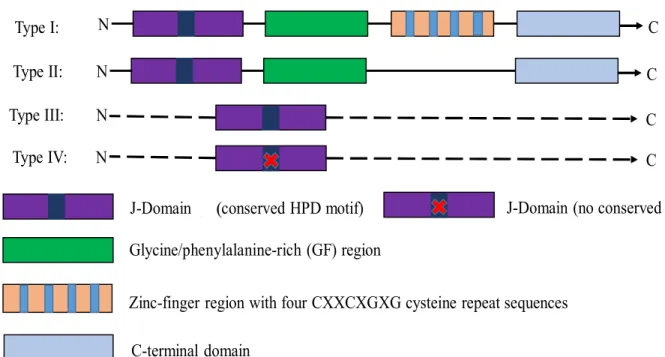
26 that a possible function of the non-classical type III/IV Hsp40s could be to regulate the activity of bona fide Hsp40s. Like other type IV PfHsp40s, PFF1010c also lacks the HPD motif, and its hypothesized expression at the gametocyte stage of the parasite suggests that it may be essential for parasite development.
Hypothesis
Objectives
MATERIALS
METHODS
- Bioinformatics analysis
- Preparation of competent E. coli JM109 and BL21 Star (DE3) cells
- Transformation of competent E. coli JM109 cells BL21 Star (DE3) cells
- Plasmid DNA Extraction
- Restriction Digestion of Plasmid DNA
- Peptide specific anti-PFF1010c antibody design
- Expression of PFF1010c recombinant protein in E. coli JM109 and BL21 Star (DE3)
- Determination of protein solubility of PFF1010c
- Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)…
- Western blot analysis
- Preparation of Ni 2+ Chelating Sepharose beads
- Purification of PFF1010c recombinant protein under denaturing conditions (urea
- Purification of PFF1010c recombinant protein from E. coli BL21 Star (DE3)
- Investigation of the interaction between PFF1010c and PfHsp70-1 by far Western
- Total protein isolation from in vitro P. falciparum culture
- Investigation of the expression of PFF1010c by parasites at distinct growth stages
- Investigation of the expression of PFF1010c by heat stressed asexual parasites
- Investigation of the expression select heat shock proteins by parasites at the gametocyte
The samples were boiled for ten minutes and analyzed by 12 % SDS-PAGE and Western blot analyses. The cells were stored overnight at -80 ˚C and thawed the next morning, followed by mild sonication at amplitude setting of 50 for 5 cycles with 15 second pulse and 30 second pause after each cycle. The samples were analyzed by 12% SDS-PAGE and Western blot analysis to determine the solubility of recombinant PFF1010c.
Cells expressing recombinant PFF1010c protein after induction in 1L 2 x YT broth culture containing 100 mg/ml ampicillin for 4 h at 37 °C, cells were harvested by centrifugation at 5000 g for 20 min at 4 °C and pelleted again suspended in denaturing lysis buffer (300 mM NaCl, 10 mM imidazole, 100 mM Tris, pH 8.0, 1 mM PMSF, 1 mM lysozyme, and 8 M urea). Cells were stored overnight at -80 ºC and thawed the following morning, followed by mild sonication at an amplitude setting of 50 for 5 cycles with a 15 s pulse and 30 s pause after each cycle and centrifuged at 5000 g for 20 min at 4 ºC. Proteins were separated using 12% SDS-PAGE and Western blot analysis was used to confirm the presence of eluted protein.
38 was resolved using 12% SDS-PAGE and Western blot analysis was used to confirm the presence of the eluted protein. The expression of PFF1010c by parasites at different stages was confirmed by Western blot analysis using anti-PFF1010c antibody. The expression of PFF1010c by heat-stressed asexual stage parasites was examined by Western blot analysis using anti-PFF1010c antibody.
RESULTS
Bioinformatics
- Protein sequence alignments…
- Comparison of 3 dimensional models of PFF1010c, DnaJ and Ydj1
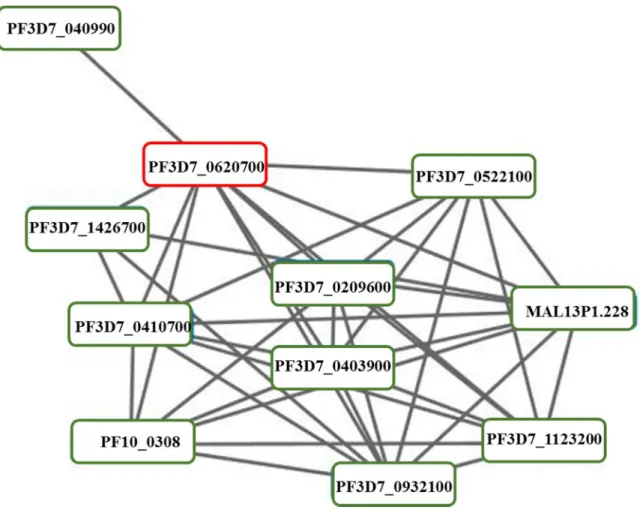
- Prediction of PFF1010c’s interaction partners
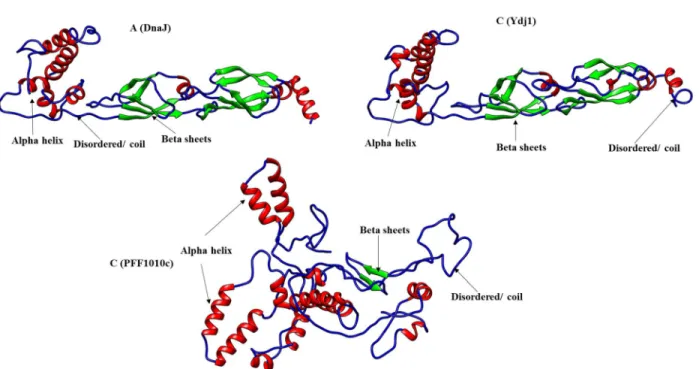
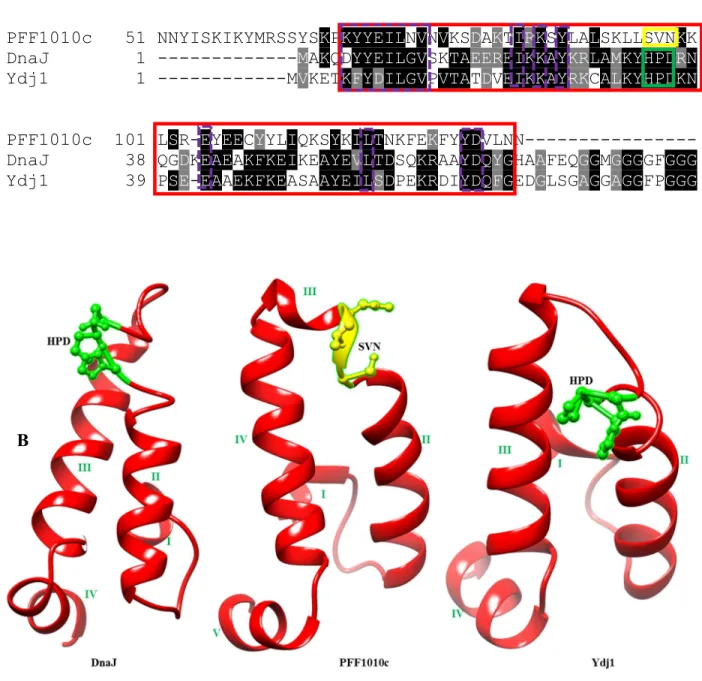
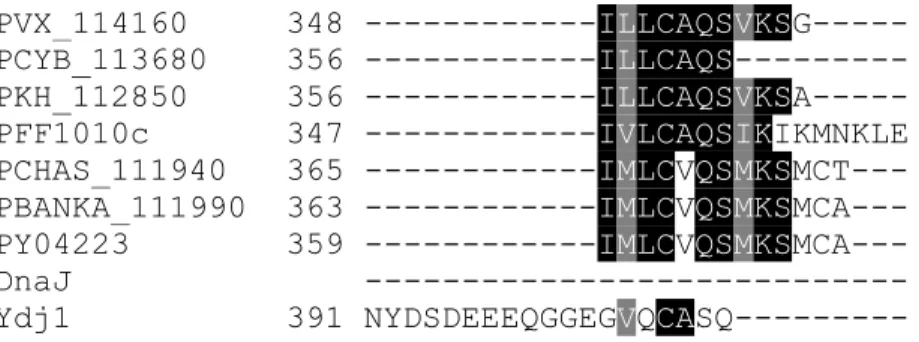
The multiple sequence alignments showed the presence of an insertion on DnaJ and Ydj1 the G/F rich region (Figure 3.1.1; purple box) which is absent in PFF1010c and its Plasmodium homologs, the G/F rich region is ' a disordered region that has been suggested to provide flexibility to these proteins (Karzai and MaMacken, 1996). The 3D model of PFF1010c, DnaJ, and Ydj1 was generated using Phyre2 and visualized using UCSF Chimera ( www.cgl.ucsf.edu/chimera ; Pettersen et al., 2004 ). The 3D model of PFF1010c was compared to that of DnaJ and Ydj1 using fitting tools on Chimera (Figure 3.1.2).
PFF1010c appears to be in a more compact conformation than DnaJ and Ydj1, which are in a more relaxed conformation (Figure 3.1.2). The J-domain models of PFF1010c, DnaJ and Ydj1 were compared by both multiple sequence alignment and also by their 3D models (Figure 3.1.3). Sequences of PFF1010c, DnaJ and Ydj1 were aligned and an extract of multiple sequence alignment highlighting the J domain is represented by a blue solid box (Figure 3.1.3A).
There are several residues that are conserved between PFF1010c, DnaJ and Ydj1's J domain (Figure 3.1.3; purple dashed boxes). The models were generated by Phyre2 ( www.sbg.bio.ic.ac.uk/phyre2 ) and visualized using Chimera ( www.cgl.ucsf.edu/chimera ; Pettersen et al., 2004 ). A) An excerpt from a multiple sequence alignment showing the J domains of PFF1010c, DnaJ and Ydj1 (highlighted by the blue solid box) (Hennessy et al., 2005). Conserved residues within the J domains (highlighted by purple dashed boxes). B) 3D models of DnaJ, PFF1010c and Ydj1 with the HPD motif in DnaJ and Ydj1 represented by ball and stick in green and SVN of PFF1010c also represented in ball and stick in yellow.
Confirmation of pQE30/PFF1010c plasmid
53 observed in lane 4 was approximately 3424 bp and the smaller fragment was just above 1089 bp, these fragments were therefore of the expected bp sizes.
Expression of PFF1010c recombinant protein
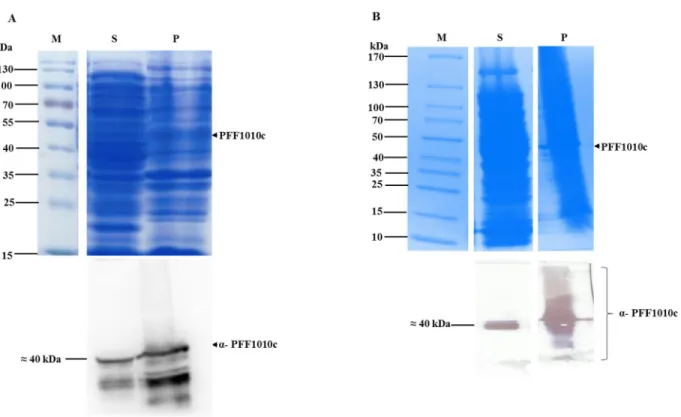
The pre-induction sample shows no expression of PFF1010c on SDS-PAGE (Figure 3.3A; lane 0; upper panel). However, Western blot analysis using anti-PFF1010c antibodies detected PFF1010c in the pre-induction sample (Figure 3.3A; lower panel), revealing leaky expression. Western blot analyzes using a peptide-specific antibody against PFF1010c also detected a second band under PFF1010c, which is most likely a degradation product and not due to non-specific binding, as the band was not present in control or pre-induction samples (Figure 3.3A, lower panel).
Western blot analysis using anti-His and anti-PFF1010c revealed leaky expression as PFF1010c was detected in the pre-induced sample (Figure 3.3B; Lane 0; lower panel). SDS-PAGE (upper panel), and Western analyzes of (lower panel) the expression of PFF1010c using anti-His antibody and anti-PFF1010c antibody, respectively. Lane M – PagerRuler Prestained Protein Ladder (Thermo Scientific, USA) in kDa is shown on the left; lane C – the total extract for cells transformed with pQE30 plasmid; lane 0 – total extract for cells transformed with pQE30/PFF1010c before IPTG induction; lanes 1, 2, 3 and 4 – total cell lysate obtained 1, 2, 3 and 4 hours after induction.
Solubility studies of PFF1010c recombinant protein
Protein purification was performed batchwise, under denaturing conditions in the presence of PEI for protein solubilization, as previously described by Botha and colleagues (2010).
Purification of PFF1010c recombinant protein
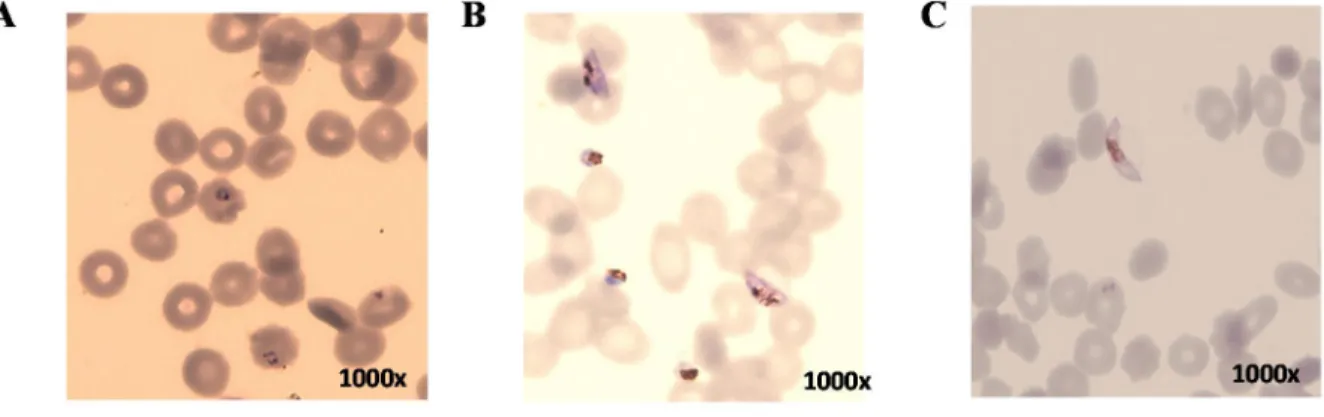
Western blot analysis was performed using peptide anti-PFF1010c antibody to confirm the expression of PFF1010c in the early and late gametocyte stages of the malaria parasite life cycle (Figure 3.7.2B). PFF1010c appears to be poorly expressed as indicated by the intensity of the Western blot analysis (Figure 3.7.2B). (A) 12% SDS-PAGE of the analysis of malaria parasites with PFF1010c expression at different stages of the parasite life cycle.
However, expression of the protein at the gametocyte stage has not been confirmed to date. This confirmed that PFF1010c is only expressed at the gametocyte stage of the parasites. An investigation of the expression of these proteins at the gametocyte stage was performed to determine which are co-expressed with PFF1010c.
In conclusion, this study confirmed that PFF1010c is expressed in the early and late stages of the gametocyte phase of parasite growth. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Structural insights into the inactive subunit of the apicoplast-localized caseinolytic protease complex of Plasmodium falciparum.
Effect of heat shock on the viability, growth and expression of the heat shock protein 'PFHSP70-I' gene from Plasmodium falciparum. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machinery.
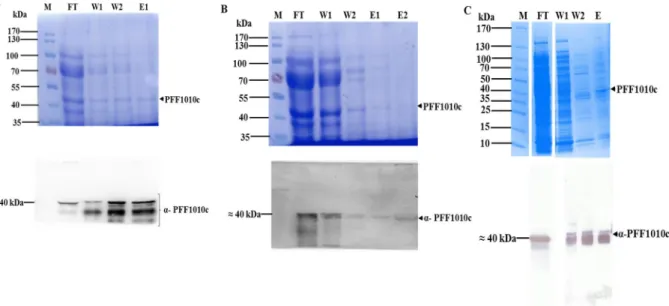
Detection of PFF1010c-PfHsp70-1 interaction using far Western analysis
Investigating the expression of PFF1010c by parasites at distinct growth
Proteins have been isolated from parasites at different stages of the parasite's life cycle; asexual stage, early gametocyte stage and the late gametocyte stage as previously described (Zininga et al., 2015). These febrile conditions are known to induce select Hsps that are required for maintaining the development of the parasite (Pallavi et al., 2010). In addition, the upregulation of some of the Hsps during febrile conditions augments parasite infectivity (Pallavi et al., 2010).
To gain insight into the structure-function characteristics of PFF1010c, bioinformatics analyzes were performed. Recognizing the potential difficulties of expression of a malaria protein in a bacterial host, this study used two E. Leaky expression from the lac promoter can be minimized by introducing a catabolite such as glucose into the growth medium (Saluta and Bell, 1998 ).
72 Expression data of PFF1010c assayed by oligonucleotide microarray analysis indicate that the protein is expressed in the gametocyte stage of the parasite's life cycle (www.PlasmoDB.org; . Aurrecoechea et al., 2009). PfHsp70-1 was confirmed to be expressed at both the early and late gametocyte stages of the parasite, while PfHop and PfHsp90 were confirmed to be expressed only at the early gametocyte stage of the parasite's development (Figure 3.8). Attempts to purify the recombinant protein were unsuccessful, thereby hindering further biochemical characterization of the protein.
Molecular chaperones and protein quality control, Cell 125 443–. Dedication of the malaria parasite Plasmodium falciparum to sexual and asexual development. Co-expression of the Plasmodium falciparum molecular chaperone, PfHsp70, enhances the heterologous production of the antimalarial target GTP-cyclohydrolase I, PfGCHI.
Investigating the expression of select heat shock proteins by parasites at the gametocyte
Assessment of PFF1010c’s expression at the asexual stage
Discussion and conclusive remarks
However, type IV Hsp40 has been proposed to exert its function through different mechanisms than type I and type III Hsp40 (Botha et al., 2007). Comparison of the 3D structures of PFF1010c, Ydj1 and DnaJ revealed that the two Hsp40 type I have very similar protein structures. Purification under native conditions was used because the protein was partially recovered in the soluble fraction after separation of the soluble and insoluble fractions by centrifugation (Figure 3.4B).
Protocols to improve the production and purification of the recombinant protein need to be developed to facilitate further biochemical characterization of this apparently important molecule. Insights into the structural dynamics of the Hsp110-Hsp70 interaction reveal the mechanism for nucleotide exchange activity. Asymmetry of the GroEL-GroES complex under physiological conditions as revealed by small-angle X-ray scattering.
A conserved HPD sequence of the J domain is required for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. The top panel shows the hydrophobicity plot of the total PFF1010c and the bottom panel shows the hydrophobicity plot of the selected regions.