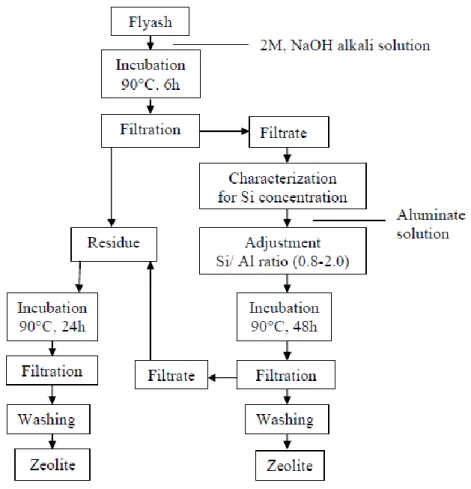
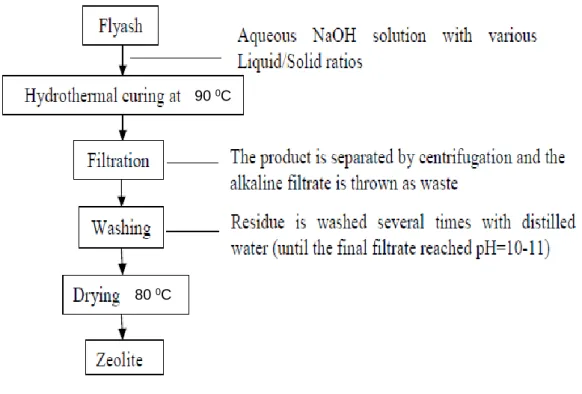
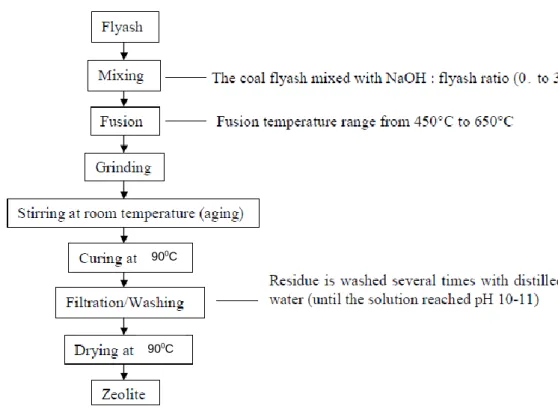
Coal fly ash waste produced by the combustion process of coal is of increasing concern. It was discovered that one of the useful materials that can be synthesized from fly ash is zeolites. Therefore, many studies have been conducted on optimizing the synthesis of various zeolites from coal fly ash.
It was found that using fusion as a pre-synthesis step can extract 26% of the silicon within the initial fly ash. Coal fly ash, NaOH, and water were fed into the jet cycle pilot plant at a 1:1:5 mass ratio of fly ash:NaOH:water. Available: http://www.intechopen.com/books/zeolites-use-minerals/upscaling-of-zeolite-synthesis-from-coal-fly-ash-waste-current-status-and- future-outlook.
Introduction
- Background
- Problem statement
- Objectives and research questions
- Significance of study
- Research approach
- Delineations
- Thesis outline and structure
Another challenge was the inability to develop a process for the pre-scale optimization of the zeolite. The aim of this study is to investigate an alternative method to improve the dissolution of Si and Al from a South African coal fly ash for the synthesis of faujasite zeolites with the potential to scale up the process. What is the effect of the optimized synthesis conditions on the distribution fate of elements.
The research was based on the study conducted by Muysoka (2012), who used this study as a base case reference for the synthesis of the hierarchically structured zeolite X. Therefore, the study began with the synthesis of the hierarchically structured zeolite X from the fusion method. The next phase of the experiments involved modifying the presynthesis method, namely the fusion method, with a jet loop system and low temperature curing.
Literature review
- Coal fly ash
- Introduction
- Mineralogy and chemical nature of coal fly ash
- Morphology of coal fly ash
- Classification of coal fly ash
- Environmental impacts of coal fly ash
- Applications of coal fly ash
- Zeolites
- Introduction
- Structural framework of zeolites
- Properties and applications of zeolites
- Synthesis of zeolites
- Synthesis of zeolites from coal fly ash
- Mechanism of zeolite formation
- Factors affecting zeolite formation
- Characterisation techniques
- Hydrodynamic cavitation
- Material balances
- Gaps in the literature concerning scale-up of zeolite synthesis
- Chapter summary
CFA is one of the most complex anthropogenic substances that can be classified based on the diversity of its constituents (Blissett and Rowson, 2012; Yao et al., 2015). One of the dominant uses of CFA in large quantities is in the cement industry for the production of concrete (Ahmaruzzaman, 2010; Yao et al., 2015). This is due to the low adsorption capacity of CFA, which depends mainly on the origin of CFA and the chemical treatment used (Ahmaruzzaman, 2010; Ahmaruzzaman, 2011; Yao et al., 2015).
CFA is converted into zeolite by alkaline hydrothermal reaction of CFA-containing aluminosilicates, as mentioned earlier (Bukhari et al., 2015). In a review by Bukhari et al. 2015) of interesting nature shared by zeolite P and habazite as a whole. This is due to the increase in the rate of dissolution of crystalline and amorphous substances with increasing water content (Querol et al., 1995; Querol et al., 2001).
The concentration of the light-absorbing substance is given by c, while di is the path length of the sample in cm (Perkampus et al., 2013). Some of the methods were highlighted by zeolite synthesis from CFA and the formation mechanism was discussed.
Experimental
- Experimental approach overview
- Materials and chemicals
- Experimental approach for base case zeolite X synthesis
- Preparation of alkali fused fly ash
- Synthesis of zeolite X from fly ash
- Material balance for zeolite X synthesis from fusion process
- Experimental procedure for jet loop pre-synthesis
- Experimental set-up and materials
- Effect of jet loop mixing time and curing of samples
- Material balance around jet loop system
- Characterisation techniques
- Elemental analysis
- Mineralogical characterisation by XRD
- Physical characterisation by morphological analysis
- Structural analysis
- Chapter summary
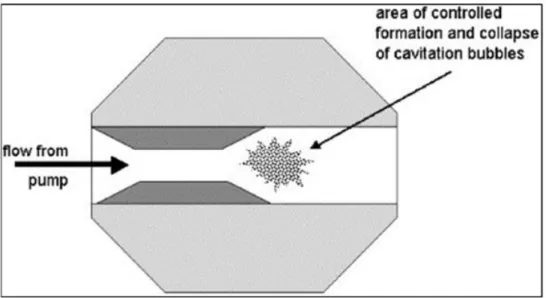
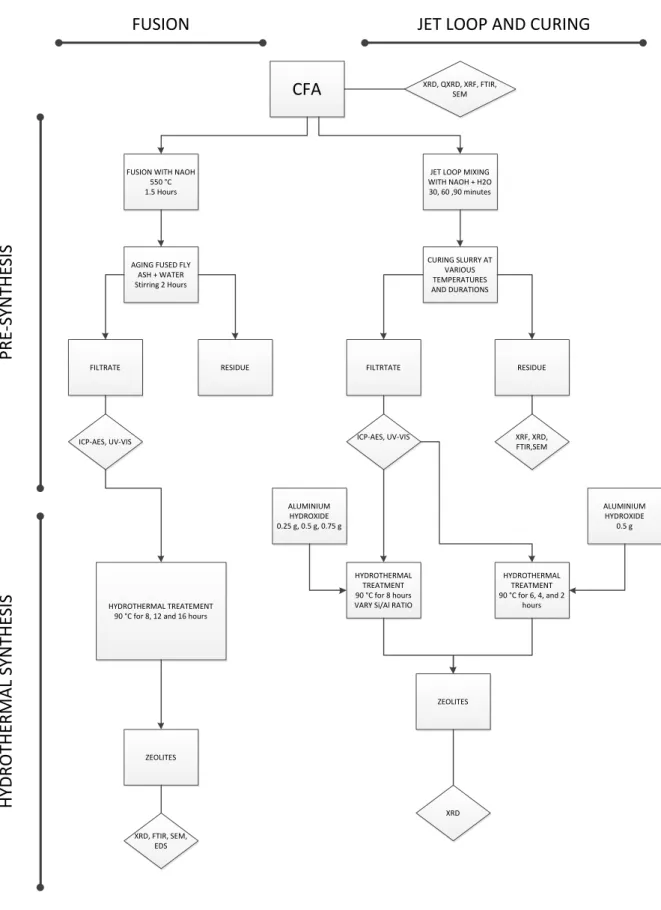
A life cycle system was used to replace the high temperature melting process used in the previous case study. It involved an experiment to determine the optimum jet loop mixing time and baking time. A mass balance was also performed around the jet loop as a comparison to that performed by the fusion method.
An attempt was then made to synthesize zeolite X from the jet loop filtrate produced after curing and further mixing of the jet loop slurry. The jet loop has also been used in a study by Nyale (2014) for the synthesis of geopolymers from South African fly ash. Once the optimal jet loop mixing time and curing time were determined, the hydrothermal synthesis of the produced filtrate was performed under the same conditions, optimized during melting (90°C for 8 hours).
Samples produced in the jet loop and solidification phase of the experiments are listed in Table 3-3 along with the codes used for the samples in the experiments. The material balance for the entire jet loop and the solidification and hydrothermal treatment process was performed based on separate unit balances for each process stage. The slurry produced was then weighed to determine the overall material balance around the jet loop process and quantify the losses incurred in the process.
A balance was then made around the amount of slurry produced from the jet loop that could be cured with the curing process. The curing process was carried out in plastic containers and the total volume of slurry produced from the jetloop pilot plant could not all be cured. First, a 100 ppm Si standard was made by adding 5 ml of the 1000 ppm stock solution to a 50 ml volumetric flask and making up with deionized water.
5 mL of the 100 ppm Si standard was then added to another 50 mL plastic volumetric flask and made up to volume with deionized water.
Characterisation of starting material and base case study
- Introduction
- Characterisation of starting material
- Base case study of zeolite X synthesis from fusion method
- Material balance for base case study of zeolite X synthesis from fusion method
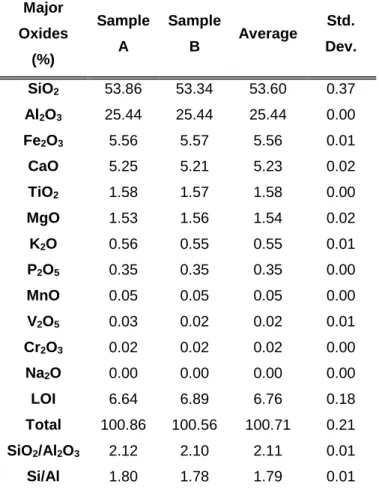
It can be seen that CFA mainly consists of an amorphous glassy phase, which is the most reactive phase and mainly determines the type and composition of the resulting zeolite. The smoothness of the particles, which can be seen up close in Figure 4-4(d), is due to the mineral particles being covered by an amorphous glassy phase (Inada et al., 2005b; Musyoka, 2012). Results presented here for a base case study of a fusion method for the synthesis of a novel hierarchical morphology of zeolite X from CFA using fusion presynthesis.
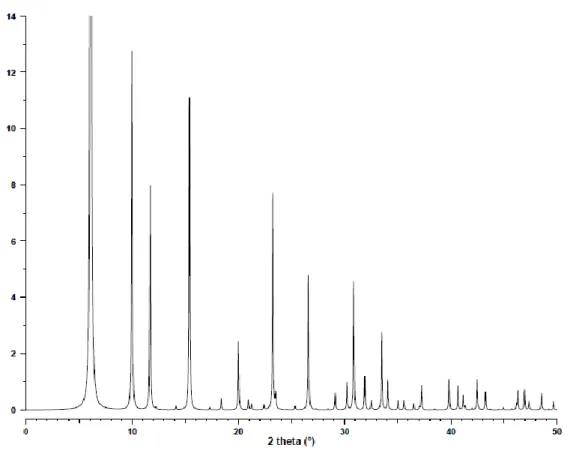
Due to the different nature of the starting fly ash, it was necessary to optimize the synthesis of zeolite X. This is due to the high purity of the phase and the intensity of the XRD peak found at 6.1 degrees 2𝜃, together with most of the peaks attributed to zeolite X. This is quantitatively shown in the table 4-3 in terms of phase crystallinity of mixed phase zeolites, where zeolite X has 66.85% phase crystallinity.
The following Figures 4-7 show the SEM micrographs of the zeolites obtained at the varying hydrothermal synthesis times and at 90°C. The following table 4-5 shows the results of the EDS analysis performed in addition to the SEM analysis of the base case fusion results. The next section deals with the material balance performed around the best result of the synthesis in the base case (8 hours at 90°C) to determine the fate of the elements in this synthesis.
It can be seen from Figure 4-8 that the efficiency of the synthesis process with respect to the yield of zeolite produced from the amount of initial CFA used is extremely poor producing only 0.11 g of zeolite. A general material balance was produced for the base case fusion synthesis of optimal conditions. The following chapter will address the replacement of the melting process with a jet lag system, including solidification of the produced slurry.
This will serve as a comparison with the base case melting process in an attempt to increase the yield of zeolite obtained by increasing the dissolution of silicon and aluminum from the initial CFA.
Jet loop assisted pre-synthesis
- Material balance around jet loop and curing process
- Synthesis of zeolite X from the jet loop and cured filtrate
- Chapter summary
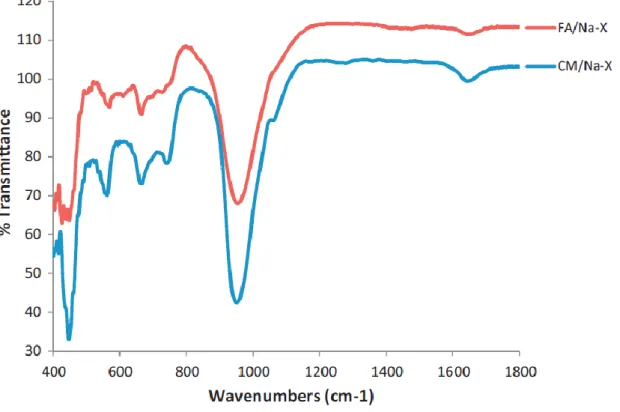
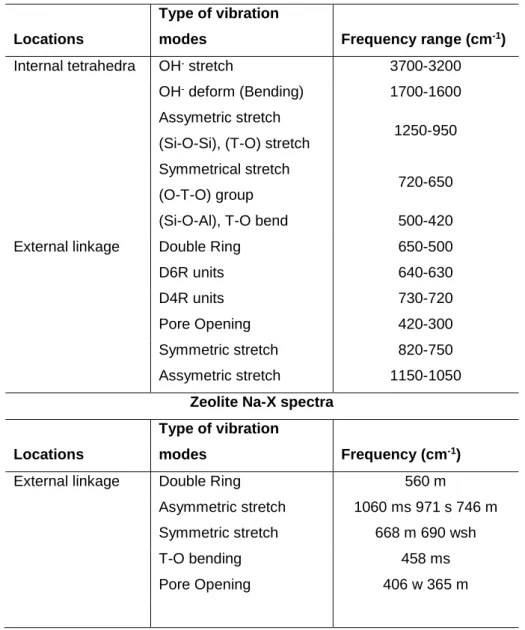
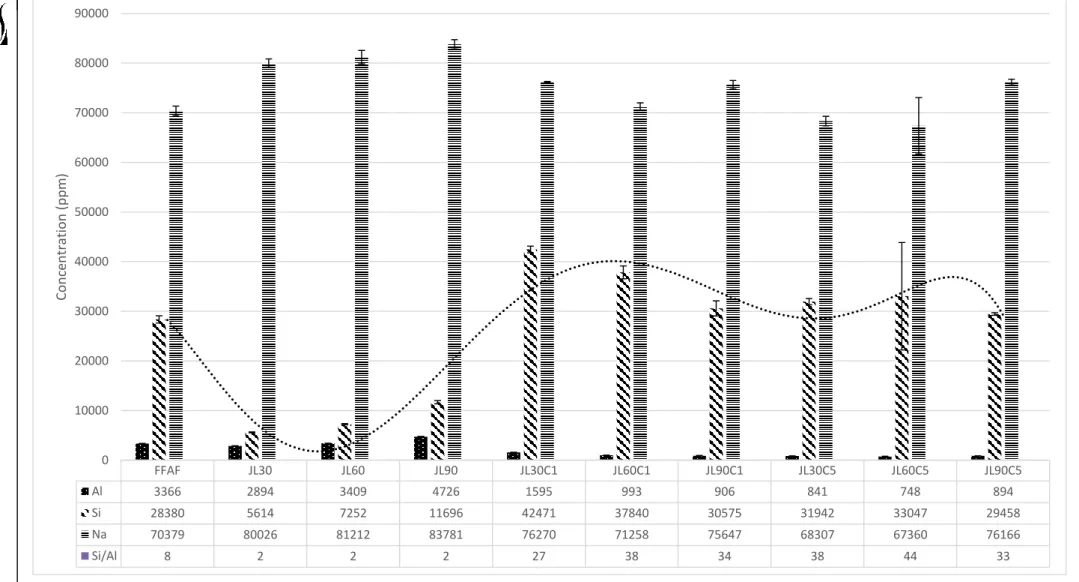
In further characterization of the jet-loop and curing presynthesis method, the solid residues separated from the filtrates were analyzed. These results thus show that curing the jet loop slurry allowed for greater dissolution of silicon in the filtrate. The FTIR spectra of the residues collected from the jet loop experiments are shown in Figure 5-8.
This is also consistent with the filtrate analysis showing the highest dissolution of silicon after curing the slurry. The lower temperature curing at 800C favors more dissolution of the silicon in the filtrates as seen in the ICP – AES results of the filtrates. As can be seen in Figure 5-9, the jet loop mixing and curing can be seen as comparable to the fusion of the fly ash with NaOH at 550 0C with respect to the breakdown of the fly ash as seen in the similar morphologies of the residues.
A material balance was performed in sections around each of the processes throughout the jet loop mixing, solidification and hydrothermal treatment. However, aluminum extraction using the jet loop and mixing process is poor, aluminum extraction only from CFA under optimal conditions. Based on ICP analysis, most elements appeared to generally report a solid residue.
Then, the remaining balance was assumed to report to the residue, based on ICP analysis of the filtrate. Based on the results of the analysis of the filtrates in the previous section, it was calculated that approx. 0.5 g of Al(OH)3 to the extracted filtrate after applying the optimized conditions of the jet loop and the curing process to resemble. The Si/Al ratio found in the fusion presynthesis filtrate. What had a significant impact on the silicon solution was the curing of the slurry produced by jet-loop mixing.
Most elements appear to report as a solid residue based on ICP – AES analysis of the filtrate. The XRF analysis of the residue deviated significantly from the expected value based on the ICP – AES results for the filtrate. Along with full recovery of solids from the jet loop and solidification process, with only 50% solids recovered from process spills and residues.
Conclusions and recommendations
- Introduction
- Conclusions
- Recommendations for future work
Distribution fate of elements during the synthesis of zeolites from South African coal fly ash. Waste minimization protocols for the zeolite synthesis process from South African coal fly ash. Effects of Stepwise Change of Heating Source on the Synthesis of Zeolite 4A from Coal Fly Ash.
Synthesis of zeolites Na-P1 from South African coal fly ash: Effect of impeller design and agitation. Large-scale synthesis of artificial zeolite from coal fly ash with a small charge of alkaline solution. Ultrasound-assisted synthesis of zeolite A from coal fly ash using mine water (acid mine drainage and circumneutral mine water) as a substitute for ultrapure water.
In situ ultrasound diagnostics of zeolite X crystallization with novel (hierarchical) morphology from coal fly ash. Microwave-assisted two-step process for the synthesis of a single-phase Na-A zeolite from coal fly ash.