However, investigators sometimes failed to adhere to the submission checklist and guidelines, resulting in delays in the timely review of protocols. Investigators should adhere to the submission checklist and guidelines to avoid delays in the ethics approval process.
INTRODUCTION AND BACKGROUND
- History of research abuses
- Obligation to obtain ethical approval
- Prominent guidance documents
- Fundamental ethical principles
- The role of ethics review boards
- Composition of IRB members
- Independence of IRBs
- Decision-making of IRBs
- Challenges with ethics review identified in the literature
- Problem statement
- Ethical review process in Tanzania
- Conceptual framework
- Objectives and research questions
- Aims
- Research questions
The guidelines therefore emphasize that committees must consider the relevance of the proposal to health care priorities within the country, scientific validity and ethical acceptability of the proposed research (McMillan & Conlon, 2004). The three widely accepted philosophical principles governing research are 1) respect for autonomy, which emphasizes the rights of an individual, 2) respect for an individual and 3) the protection of those who are incapacitated and vulnerable such as children and incompetent persons (Beauchamp, 2003; In addition, the effectiveness of IRBs has been undermined due to the IRB system's failure to adapt to the changing research environment (Christian et al., 2002).
METHODOLOGY
- Location of the study
- Brief description of study areasributing protocols
- Research methods
- Sample size and data collection process
- Data analysis
- Validity, reliability and rigour
- Ethical considerations
- Ethics review
- Participants’ rights
A mixed-methods design, if done carefully, is more powerful than a single-method design because it increases the validity of the study by confirming the results from another perspective (Silverman, 2013; Tashakkori & Teddlie, 2003; Onwuegbuzie & Johnson, 2006; Morse et al. Niehaus, 2009). Quantitative data were obtained retrospectively from the NIMR, IHI and other selected RBI databases in Tanzania (Appendix 6). This methodology was chosen due to the nature of the study, which seeks to understand temporal variability and the factors that enable or limit turnaround time for ethical review protocols.
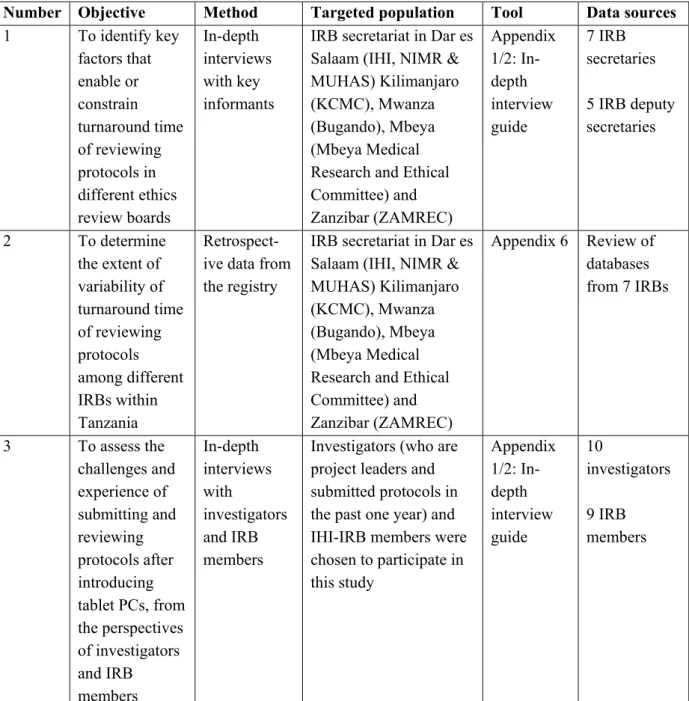
For example, in-depth interviews were conducted with selected researchers and members of the IHI-IRB to assess the experiences and challenges of submitting and reviewing protocols following the introduction of tablet PCs, from the perspectives of the researchers and IRB members respectively . Regarding the qualitative sample, seven secretaries and five assistant secretaries of the IRBs visited were involved as key informants. IHI project leaders at the time of the study who had submitted protocols the previous year were listed, and the leader from every fifth application was randomly selected to participate in the study.
Likewise, all interviews were audio-recorded and transcribed verbatim to allow for iterative review of the data to check for emerging themes and remain true to participant accounts (Noble & . Smith, 2015). Likewise, the study acknowledges limitations related to sample size, data collection, analysis, and issues related to the generalizability of findings (Sandelowski, 1993). The PI minimized intrusion by assuring participants that they did not have to participate in any aspect of the research that made them feel uncomfortable (or were informed that they could refuse to answer questions).
RESULTS
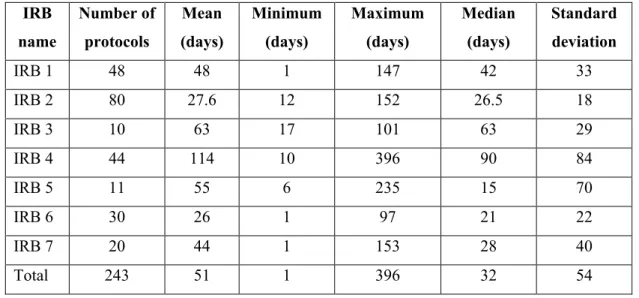
Turnaround time
Key factors that enable or constrain turnaround time of reviewing protocols
- Number of reviewers assigned to protocols
- Duration of reviewing protocols
- Decision-making processes
- Reasons for delayed feedback
- Policies and guidelines
- Training of REC members
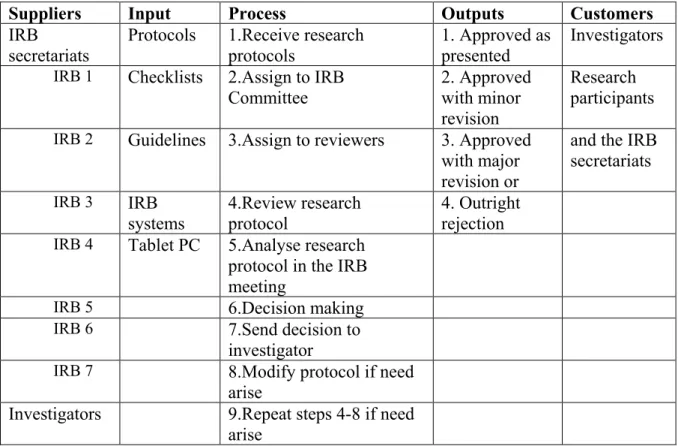
The meeting is held on the last Tuesday of the month, but there is also an accelerated evaluation.” (Secretariat, P4). However, at IHI, ZAMREC and Mbeya IRBs, the protocols were always presented to all committee members. The distribution of the submitted protocols to the reviewers varied by institution.
As explained earlier, protocols are submitted to the secretariat of the investigated institutions. Any member with a conflict of interest regarding a particular proposal may not participate in the review of the proposal and the subsequent decision-making process. Non-members such as project PIs and independent experts may be consulted as part of the review process.
In addition, lack of compensation for IRB members' time during review of protocols was also highlighted as one of the challenges. In summary, the majority of participants reported that the most frequently raised queries during the review of protocols were found in the methodology, dissemination and ICF sections. Most of the members have completed online and short term training organized by the NIMR NatREC, MUHAS and IHI.
Challenges and experiences of submitting and reviewing protocols after the
- Preferences for electronic submission
- The challenges of using tablet PCs
- Addressing barriers to review using tablet PCs
- Suggestions for improvement of the review process
It is also easy to comment and reduces the burden of carrying minutes to the meeting room and the reviewer can read minutes from anywhere. First, it is easy to carry (portable), compared to when we used to carry large files of hard copies. It's easy to share comments directly on PC; protocols can be accessed from anywhere.”
And the inconvenience of having the hard copies handed to the member, while the member is not in the office to receive them; there may be a potential for losses. The tablet computer increases the chance of getting the protocol to the reviewer, conveniently, without any error. Some members also complained about the small screen of the tablet computer and poor connection to the Internet.
For example, most members did not know how to send comments to the secretariat via tablet PCs. If you have nowhere to write, you may have nowhere to write and can come to the meeting without comment. I did not use a tablet PC to send my comments to the secretariat, but I usually share my comments during the meeting.” (Researcher and member, P2).
DISCUSSION
- Variability in turnaround time
- Factors enabling or constraining turnaround time for protocol review
- Challenges and experiences of submitting and reviewing protocols at IHI after
- Limitations of the study
In terms of procedures, most respondents who were IRB secretaries acknowledged that protocols are received and reviewed based on the institution's checklist and guidelines adapted from NatREC (Ikingura et al., 2007). In this study, it is apparent that delays related to the failure of investigators to adhere to the checklist or guidelines were reported, which is consistent with what has been reported elsewhere (Getz et al. 2011; Page & Nyeboer, 2017). . This can lead to insufficient quorum, as documented elsewhere (Kass et al., 2007), and consequently to postponement of meetings.
In general, the examination of the procedures for submitting protocols to the secretariat of the IRBs showed that they were more or less the same across IRB institutions in Tanzania and beyond (Ikingura, 2007; Kass et al., 2007) . In line with previous research findings (Kruger et al., 2014), a key challenge was that the guidelines were not updated in a timely manner. SOPs should be updated regularly, at least every five years, as there are new developments in science and technology that need to be accommodated (Fontanesi et al., 2018).
However, this was not guaranteed, as most IRBs had limited resources and training opportunities (Caligiuri et al., 2017; These findings are consistent with other studies (Hunt et al., 2016; Maskell et al. ., 2003) Electronic submission reduced the amount of paper used and associated costs and helped address some of the delay problems faced by IRBs (Hunt et al., 2016; Maskell et al., 2003; Oder and Pittman, 2015).Furthermore, this study underscores other authors' recommendations that shortening protocol review time would improve implementation of important clinical trials (Maskell et al., 2003) and time-sensitive research, thus supporting the use of electronic submissions.
CONCLUSION AND RECOMMENDATIONS
- Recommendations for investigators
- Recommendations for IRB secretariat
- Recommendations for capacity building
- Recommendations for regulators or policy-makers
- Recommendations for future research
- Conclusion
An analysis of research ethics committee decision letters: the boundary between ethics and scientific quality examined. Error rates in the process of general research applications to the Human Research Ethics Committee (Medical) at the University of the Witwatersrand: a secondary data analysis. The review notes that multicenter studies face considerable challenges, but there are strategies for obtaining institutional review board approval.
Is it the least worst we can do. 2010) Standard Operating Procedures for IHI-Institutional Review Board. Views on the process and content of ethics reviews of HIV vaccine trials among members of US institutional review boards and South African research ethics. Non-investigator-initiated projects: A retrospective analysis of the queries raised by the institutional ethics committees of a teaching hospital.
Challenges and opportunities in building health research capacity in Tanzania: a case from the National Institute for Medical. How variability in the institutional review board review process affects minimal-risk health care services. Ethics committees for biomedical research in sub-Saharan Africa: a collective assessment of their structure, functioning and outcomes.
Tool English
What are the challenges of using a tablet in the review process? Checking file access/transfer, screen reading, writing and sharing comments.). What is your overall opinion about the RBI secretariat for process improvement (submission for certification). If not, do you have plans in place to use any of the tools/platforms in the future.
Section F: Other stakeholders from COSTECH, NIMR and MUHAS to express their views on how to improve the ethics review process in the country. What is your opinion of the current regulatory framework regarding the ethical review of research involving human subjects?
Tool Swahili
Sehemu C: Lengo la 3 - Kutathmini changamoto na uzoefu wa kuwasilisha na kuhakiki itifaki baada ya kuanzishwa kwa Kompyuta ya Kompyuta Kibao, kutoka kwa mitazamo ya wachunguzi na wanachama wa IRB, mtawalia (IHI-IRB pekee). Je, una zana/jukwaa la kuwezesha shughuli za IRB (Swali kwenye kompyuta ya mkononi, programu ya Rhino, Mtandao, n.k.). Ikiwa sivyo, una mpango wowote kuhusu upatikanaji wa chombo/jukwaa hapo baadaye?Je, una bajeti ya utekelezaji wa mipango hiyo?
Nini maoni yako kuhusu mfumo wa sasa unaohusiana na maadili ya nchi kuhusu utafiti wa binadamu.
Consent form
I understand the purpose and procedures of the study Understanding Factors Enabling or Limiting Protocol Review Time in Tanzania: The Case of IRBs in Dar es Salaam, Mbeya, Mwanza, Kilimanjaro and Zanzibar Regions and I have been given an opportunity to ask questions about the study and have answered them to my satisfaction. I declare that my participation in this study is entirely voluntary and that I may withdraw at any time without affecting any treatment or care to which I would normally be entitled. I have been informed that there will be no compensation or medical treatment and costs associated with the study.
If you have any questions or need clarification at any time before signing the consent form or during the study period, please do not hesitate to ask me.
IHI-IRB application checklist
NIMR application checklist: (a) New proposal/amendment
Data Extraction Tool