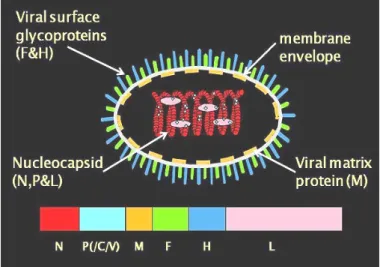
Morbilliviruses are highly infectious pathogens that cause some of the most devastating viral diseases in humans and animals worldwide (Murphy et al., 1999). It has received growing attention due to its wide distribution, economic impacts (Lefèvre and Diallo, 1990) and the role it plays in complicating the ongoing global eradication of rinderpest and epidemiological surveillance programs (Couacy-Hymann et al., 1995 ). Transmission requires close contact between infected animals in the febrile stage and susceptible animals (Braide, 1981) because of the lability of the virus outside the living host.
The discharge from eyes, nose and mouth, as well as loose stools, contain large amounts of the virus. Indirect transmission seems unlikely given the low resistance of the virus in the environment and its sensitivity to lipid solvent (Lefèvre and Diallo, 1990). The acute form of the disease was observed in WAD goats, while the WALL breed developed only a mild form (Diop et al., 2005).
There are considerable differences in the epidemiological pattern of the disease in the different ecological systems and geographical areas. One to two days after fever sets in, the mucous membranes of the mouth and eyes. After entering the virus through the respiratory system, it first localizes replication in the pharyngeal and mandibular lymph nodes as well as tonsils.
In the posterior part of the colon and rectum, discontinuous stripes of congestion (zebra stripes) form on the tops of the mucosal folds.
Treatment and vaccination
They may be dry with erosions on the gums, soft and hard palate, tongue and cheeks, and esophagus. The lung is dark red or purplish with areas that are firm to the touch, mainly in the frontal and cardiac lobes (evidence of pneumonia). Necrotizing and ulcerative lesions in the mouth and gastrointestinal tract (Roeder et al., 1994) dominate the pathology caused by PPR.
Lesions in the small intestine are generally moderate and are limited to small streaks of hemorrhage and occasional erosion in the first portions of the duodenum and terminal ileum. The colon is usually more severely affected, with congestion around the ileocecal valve, at the cecocecal junction and in the rectum. In the respiratory tract, minor erosions and petechiae can be seen on the nasal mucosa, nasal turbinates, larynx and trachea.
Bronchopneumonia may be present, usually limited to the anterior-ventral regions, and is characterized by consolidation and atelectasis. However, hyperimmune serum and supportive treatment with fluid therapy for dehydration and antibiotics to prevent secondary bacterial infection could be used to save the lives of the infected goats. Anene et al., 1987, who investigated the evaluation of the treatment of naturally occurring PPR in goats with oxytetracycline, chloramphenicol 25%.
Islam et al., 2003 stated that antibiotics combined therapy with hyperimmune serum, with an average recovery rate of 68.75%. Control of PPR in uninfected countries can be achieved using classical measures such as restriction of imports of sheep and goats from affected areas, quarantine, slaughter and proper disposal of carcasses and contact fomites and fumigation of affected premises in case of release. Immunization of small ruminants with lymph node and spleen material containing virulent virus inactivated with 1.5-5% chloroform was attempted and the animals were immune to the subsequent challenge 18 months later (Braide, 1981).
Until recently, the most practical vaccination against PPR was based on the use of tissue culture adapted rinderpest vaccine. The tissue culture rinderpest vaccine (TCRV) at a dose of 102.5 TCID50 protected goats against PPR for 12 months and the animals were unable to transmit the infection after challenge with PPR virus (Taylor, 1984 ), although the antigen was detected in tear swabs. of vaccinated animals after challenge with virulent virus (Gibbs et al., 1979). This vaccine has been used successfully to control PPR in some West African countries and is widely used in many African countries (Lefèvre and Diallo, 1990).
Secondary bacterial infection in PPR affected goat
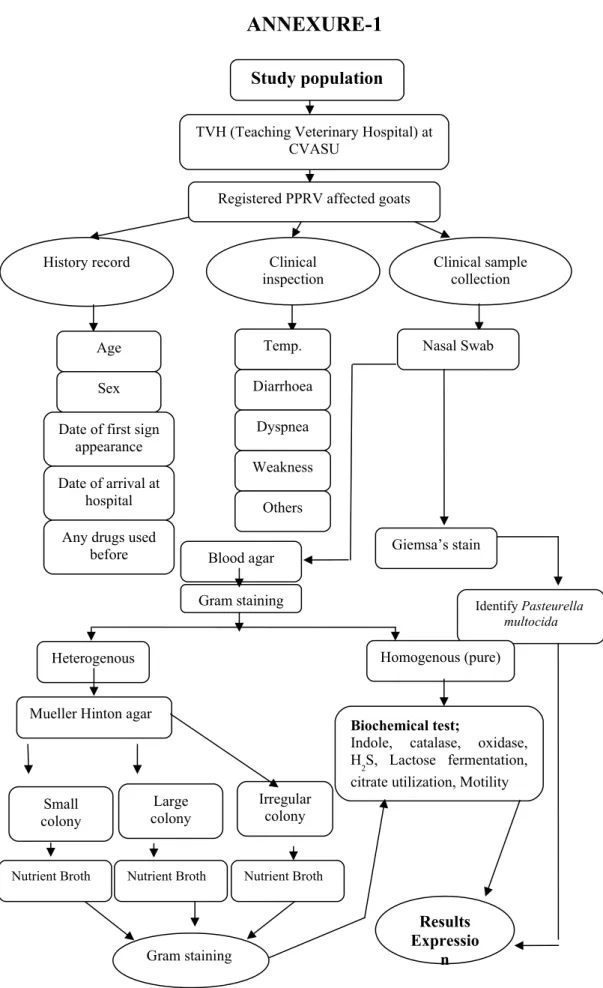
MATERIALS AND METHODS
- Duration of study
- Selection of the study area
- Selection of sample and sampling technique
- Collection of sample
- Preservation of sample
- Isolation and identification of bacteria
- Giemsa staining technique
- Biochemical analysis
- Data entry and analysis
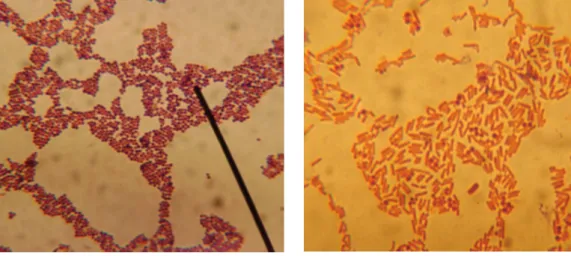
Samples collected from the nasal cavity were placed in PBS and inoculated onto ox blood agar. Blood agar plates were incubated at 37ºC overnight and then examined for growth visually for colony formation and by Gram stain technique. A pure bacterial growth was identified by having homogeneity in morphological examination under the microscope from discrete colonies.
Repeated passages on Muller-Hinton agar plates of putative mixed bacterial growth on blood agar ultimately yielded pure growth. Bacteria isolated in pure growth were identified by using various selective media for their growth and conventional biochemical tests. A bacterial film prepared from a colony was stained for 1 min with Hucker's gentian violet/crystal violet solution, which was prepared as follows: 20 ml of saturated alcoholic solution of crystal violet (2 grams of dye in 20 ml of 95% ethyl alcohol) + 80 ml of 1%.
The film was then washed with distilled water and Gram's iodine (0.33 g iodine and 0.66 g potassium iodide in 100 ml distilled water) was applied for 1 minute, followed by decolorization with acetone-alcohol (1:1) for 5- 7 seconds. Bacteria that retained a purple color were identified as Gram-positive and those that were red-stained as Gram-negative (David, 2006). The bacterial film was immersed in a diluted Giemsa stain (prepared according to the manufacturer's instructions) and the stain was kept on the film for 8-12 hours or overnight.
Confirmation of the genus, Staphylococcus was done by Gram staining and various biochemical tests, including Catalase test, Oxidase test, Methyl red, Indole, acid of different sugars, and hemolysis on Ox Blood Agar (OBA) according to the method of Cruickshank ( 1970).
RESULT
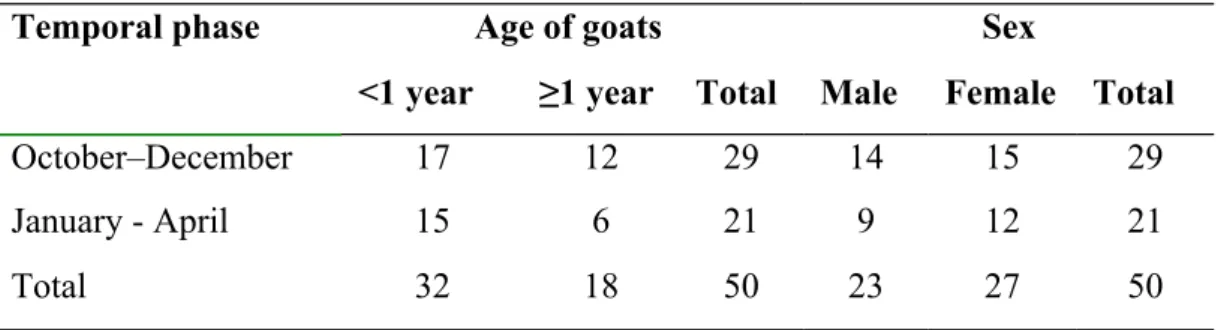
An overall statistics on the PPR-affected goats enlisted in the study
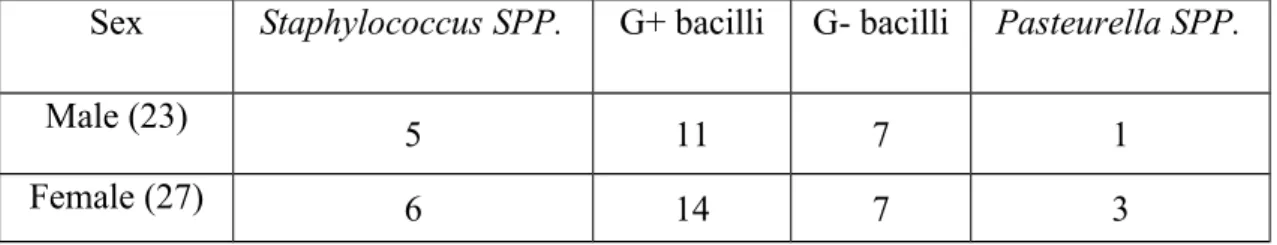
Bacterial infections in Black Bengal goats naturally infected with PPRV
There was somewhat increased number of gram-negative bacteria found in male goats forming 30% while in female goats it was 26% and shown in graph-1.
DISCUSSION
This study highlighted that the isolation of secondary bacterial infections in PPRV-infected goats differed from previous similar studies. Environmental variations (climate, weather, temperature, humidity, etc.) also play a role in this difference. We also think that frequent use of different types of antibiotics by unskilled doctors or owners for the treatment of animal diseases could develop the resistance capacity of organisms to drugs and persist in the body through various mechanisms that reduce the killing power of various immune cells .
The percentage and types of secondary bacteria isolated from PPR virus-infected goats in Chittagong district may not be representative of the overall status of Bangladesh. The growth and proliferation of different types of organisms in humid and coastal areas such as Chittagong may be a consideration. This study also suggested that detection of a specific bacterial pathogen in PPRV-infected goats could be useful to recover the animal from diseases as quickly as possible by applying selective and most appropriate antibiotics.
CONCLUSION
Evaluation of new diagnostic tools for peste des petits ruminant virus in naturally infected goat herds. Classification of peste des petits virus of ruminants as the fourth member of the genus morbillivirus. A report on the functioning of national disease reporting systems in some selected Radiscon countries with emphasis on the epidemiology of rinderpest and small ruminant disease and their potential spread through livestock trade.
ANNEXURE-1
Pictures
ANNEXURE-3 Questionnaire