This is to claim that this research work has been carried out by the author under the supervision of Dr. The unsigned examiners appointed by the Committee for Advanced Studies and Research (ClISR) hereby recommend to the Department of Metallurgical Engineering in Bangladesh Engineering University. Metallurgical) as partial fulfillment of the requirements for the diploma engineering degree (Metallurgical).
Mohor ~li, Professor, Department of Metallurgical Engineering, BUET, for his valuable suggestions, inspiring guidance and constant encouragement in all phases of the project as well as in the preparation of this thesis. Finally, the author expresses his sincere gratitude to his wife and mother-in-law for their encouragement and kind help during.. Attempts have been made to investigate the effects of niobium, nitrogen and aluminum on the structure and properties of 0.07%.
These steels, although of the ferritic-pearlite type, had a relatively high carbon content of around 0.3%. The effects of a small addition of vanadium on the structure and properties of low carbolic structural steels are NOW good.
Requirements
Bepari.9 The present review will present a brief summary of Gladman's latest theory "in the light of fer.rite gI:ain contI:ol. In equation (2.3) the critical particle radius increases as the volume fraction of particles or grains of the matrix. However, any increase in temperature causes a solution of the precipitates, and the reduced volume fraction allows grains with a smaller z-ratio to grow, thereby increasing the number of grains that grow.
The effectiveness of precipitation enhancement by microalloy additions depends on the solubility in the austenite of the precipitation phase and its precipitation kinetics during cooling. The increase in yield stress due to precipitation strength. This mainly arises from the direct interaction between dislocations and the distributed second-phase particles. orowan28 explained the cause of the decrease in yield stress when the critical particle size is exceeded. Orwan showed that the yield strength of the alloy is the sum of the matrix flow stress 'm and the resolved shear stress to bend dislocation between the dispersed particles, i.e.
The result of the l\shby-Orowan model differs quite little from that of the original. Thus, strengthening of the solid solution occurs due to deformation of the solvent lattice by the.
Materials Preparation
Measurement of Prior Austenite Grains
This technique is based on the rejection of ferrite or iron carbide at the austenite grain boundaries, which form a complete or nearly complete network in hypoeutectoid or hYPBreutectoid steel. Steels with a carbon content far from the "extratectoid composition" will reject either ferrite or cementite after slow cooling through the transformation zone, depending on the carbon content of the steel. Rejection of these constituents occurs mainly at the previous austenitic grain boundaries and, under appropriate conditions, they can break up the ingredients to form a network around the original grains.
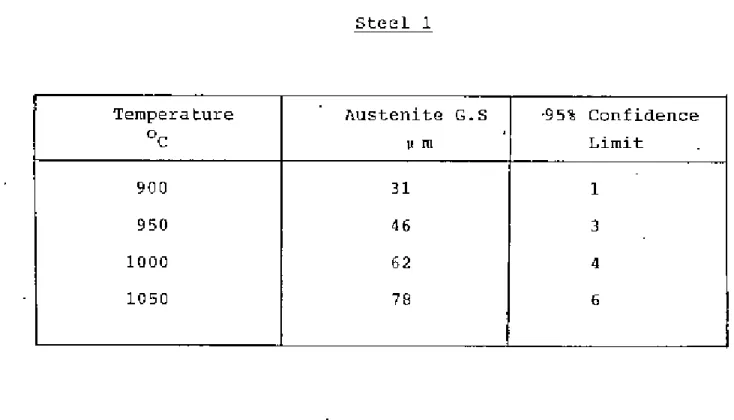
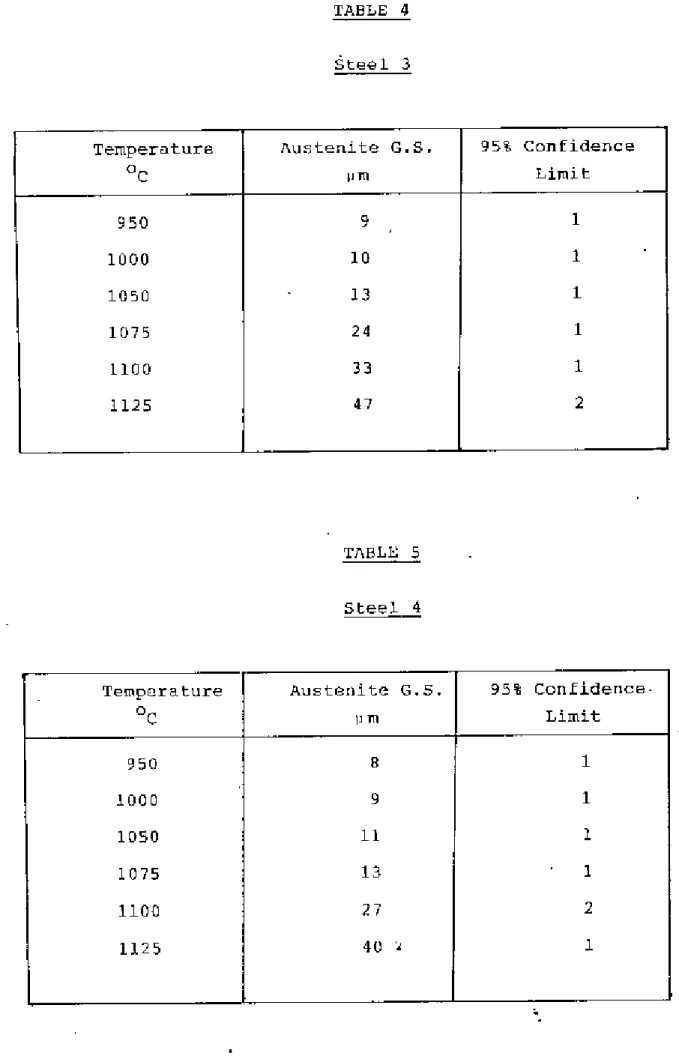
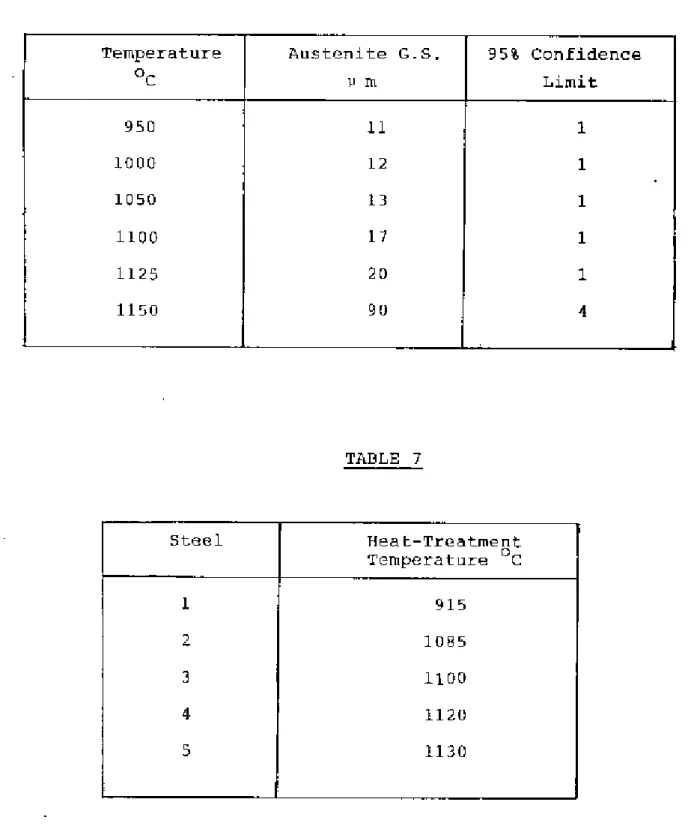
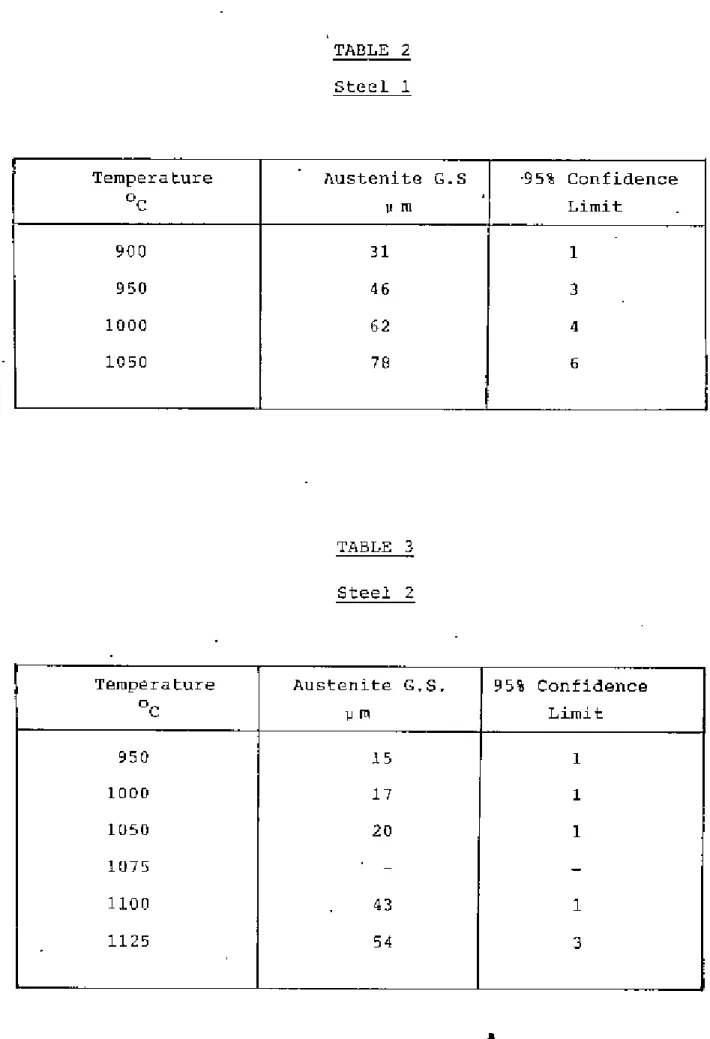
The temperature was determined by experiment and Grror and WilS were found to be between 810-860oC. The samples were kept under the above temperature. Grain size was measured using the average linear intercept method38 counting grilin boundary intersection points I>,'ithth", circumference of the circle in the eye view of a microscope". The effective circumference of the circle WOJS determined precisely by measuring its diameters with reference to a step micrometer at the magnification used. The isothermal transformation technique proved troublesome in revealing the former austenite grain boundaries of these low carbon steels and much time was spent in obtaining “satisfactory results.
The heat treatment temperature for each steel was determined by a careful examination of the austenite grain size. criteria for determining the heat treatment temperature for each steel were that the steel has the same austenite grain size and that the temperatures are such that an appl:eciab1e pl:portion of the dissolved e1"ments came into solution for subsequent precipitation The heat treatment of samples Well:e carried out in the D1ue-'M Silicon Carbide heelt treatment furnace.
Preparation and Mechanical Testing of Tensile Specimens The heat-treated 15 mm bars were then machined into
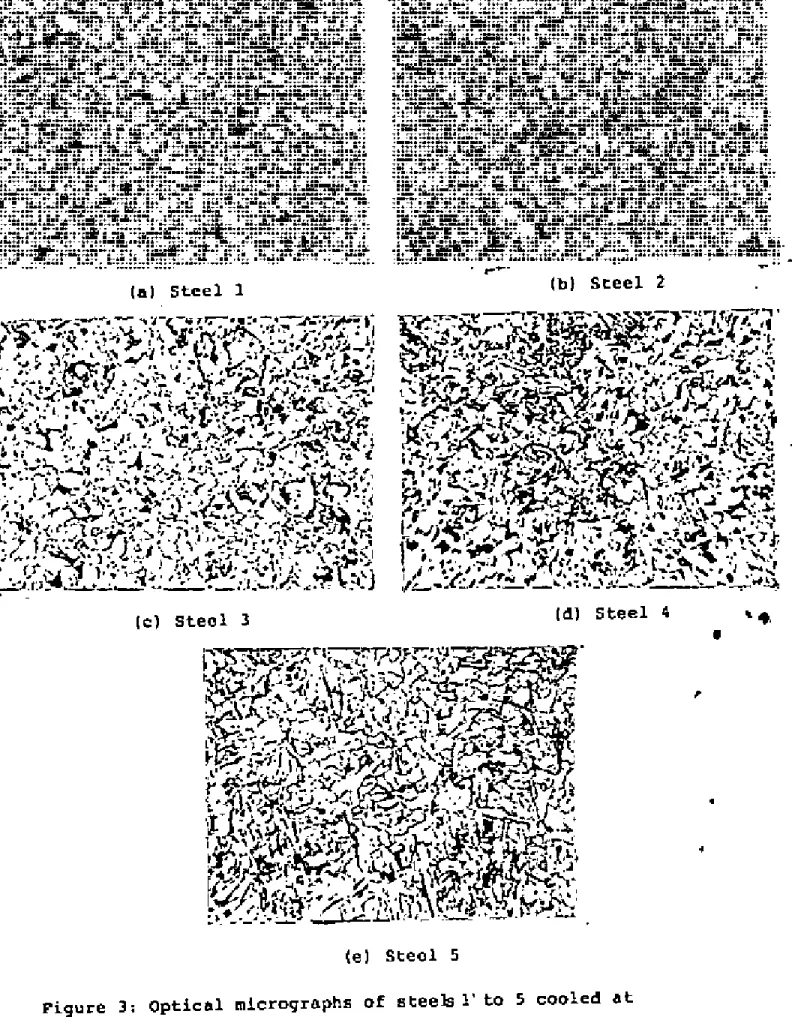
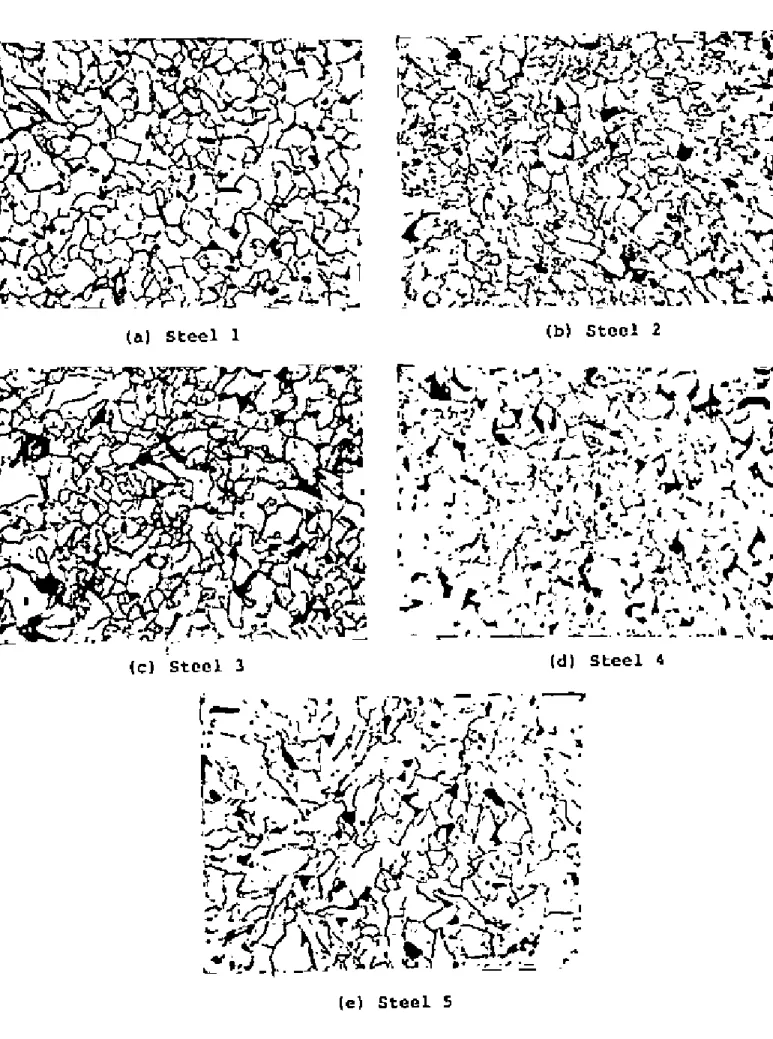
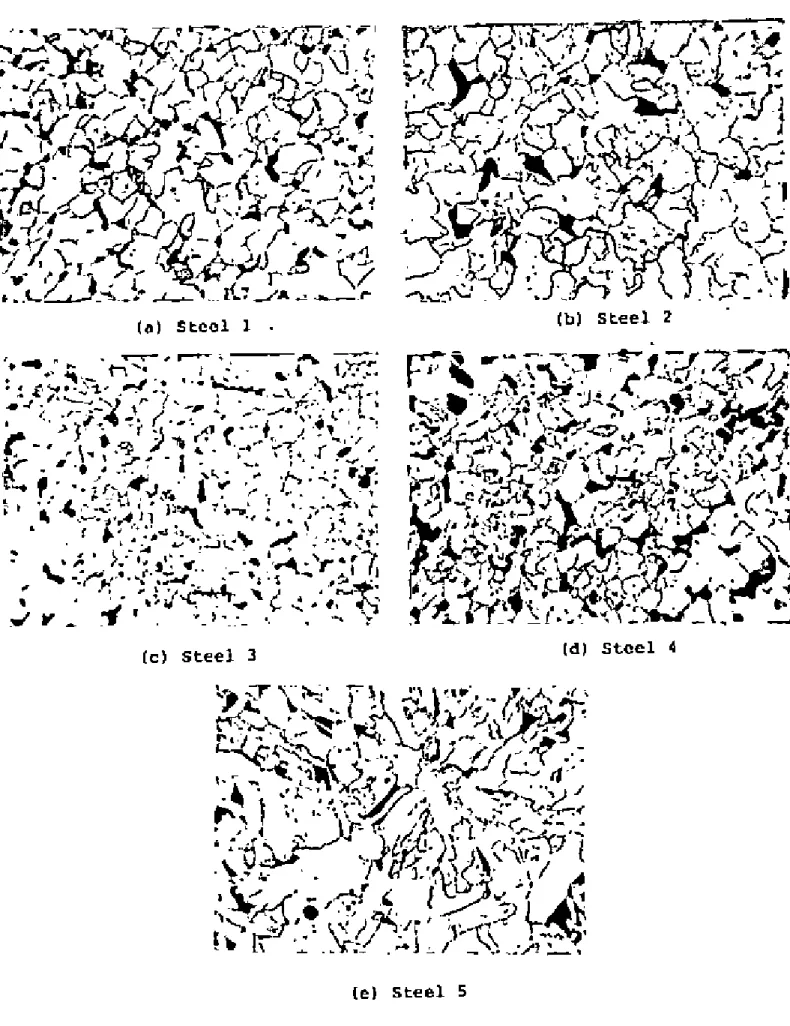
Optical Microscopv
If the fictitious ferrite grain size is ~s L, and the volume fraction of pearlite is 'v', then the actual ferrite grain size is. Although this technique proved difficult for the steels examined, satisfactory results were obtained through experimentation. The austenite grain.I!SlZe of the steels at different temperatures is shown in Tilblos 2-6 and plotted in Figure 2.
On the other hand, the austenite grain size remained very small in the temperature range above which the precipitates are effective as inhibitors.
Heat-Treatment Temperature
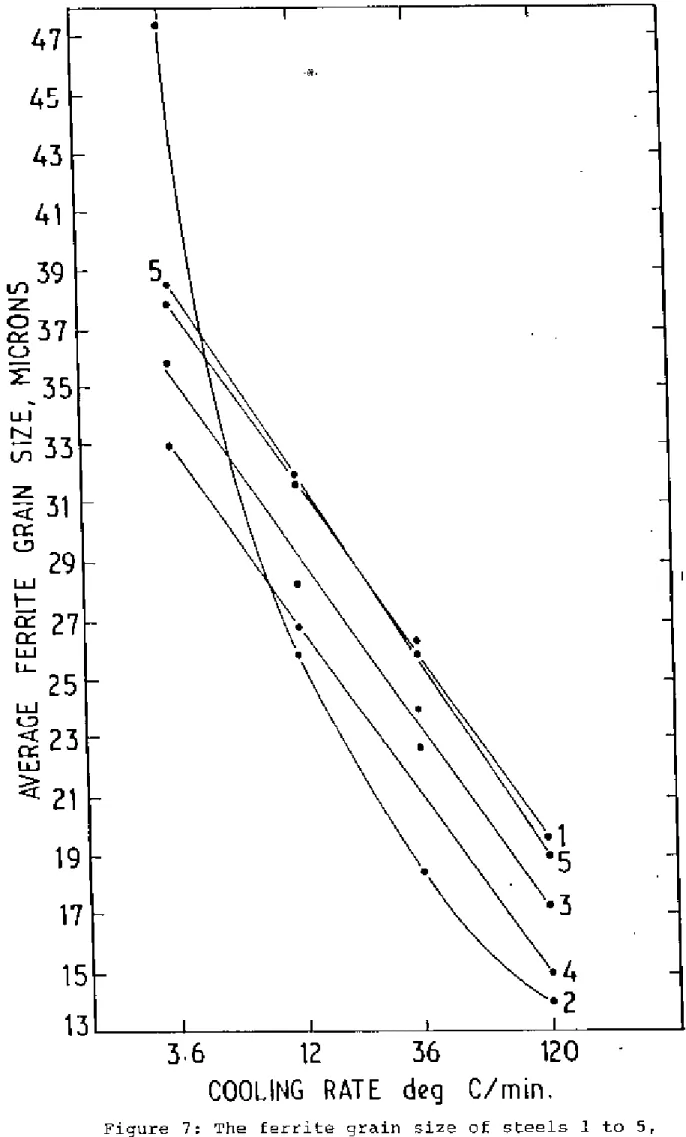
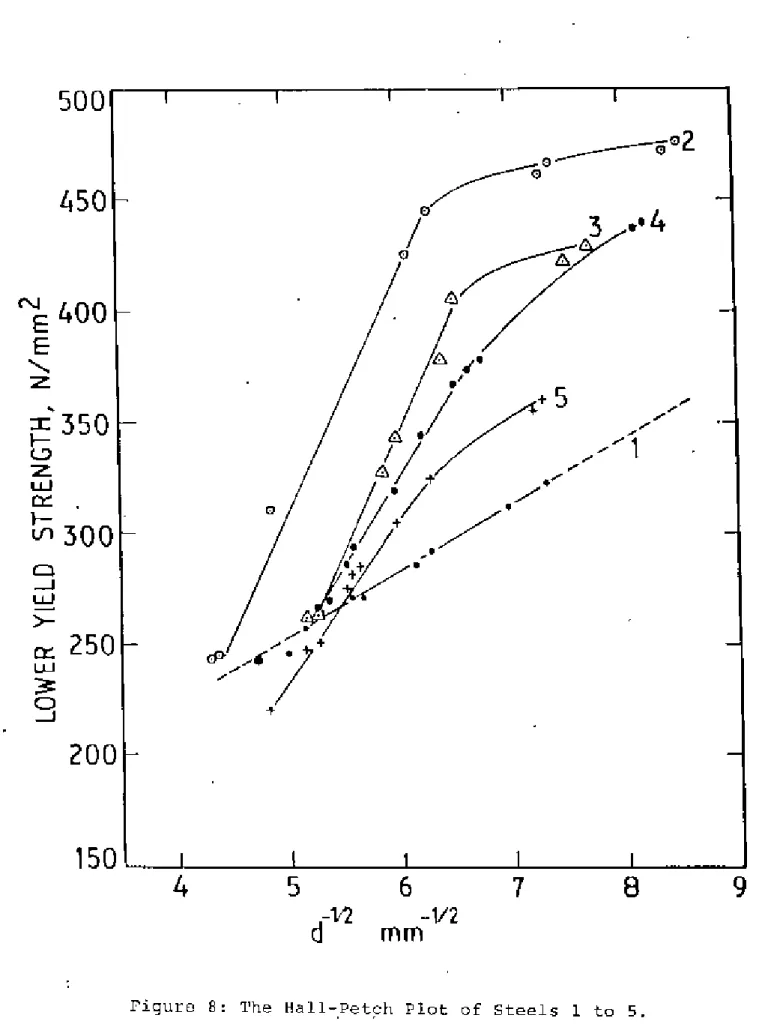
Ferrite Grain Size ilnd Hall-Petch Plot
2 at the slowest cooling rate is believed to be caused by the rapid overage of tllG NbC precipitates. It is clear from Figure 8 that the strength of steel 5 is lower than that of steel 1 at the slowest cooling rate, but it increases sharply when the cooling rate is adjusted and gradually becomes stronger than plain carbon steel 1. This procedure can give SOME small error in ferrite grain size measurement at this cooling rate.
The yield strength increment of the steels at each cooling rate was determined and listed in Table 19. It can be seen from Figure 14 that cooling rates of 1200C, 1200C and 120C per minute are the cooling rates that optimize precipitation hardening in steels 4, ) and 2, respectively. This is probably due to faster aging of Nb(C,N) particles in the absence of aluminum in steel 3 at . cooling rate.
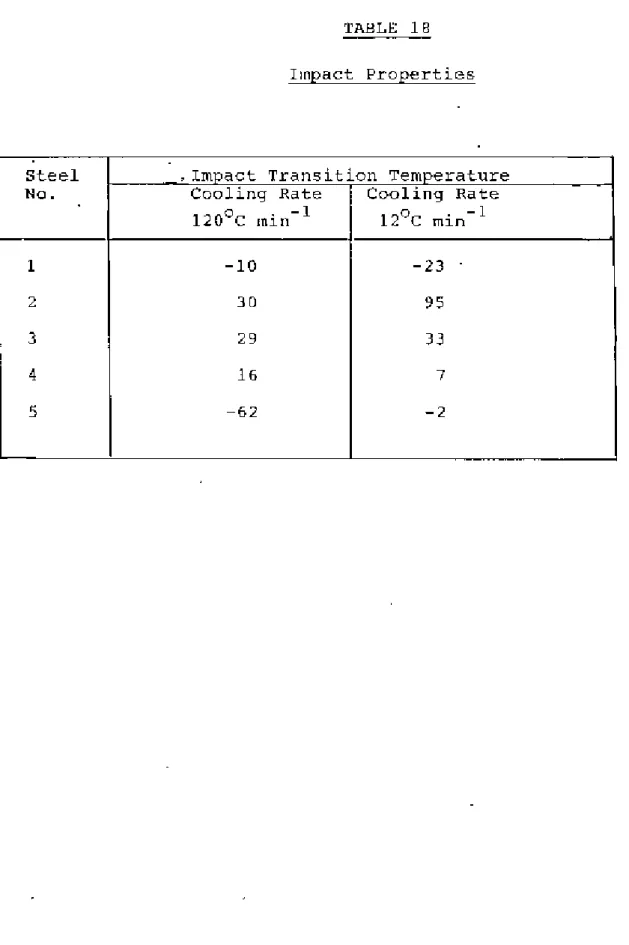
It is evident from Table 18 that for plain carbon steel 1, the impact transition temperature increases with decreasing cooling rate. decreases 8.9 and this reduction in free nitrogen content lowers the ITT The higher the ITT of steel I with the rapid cooling rate of 12(}oC per min than that with the slow cooling rate of 120C per m~ n indicates that bene - The formal effect of fine ferrite grains is overcome by.
Because the amount of soluble nitrogen present in steels during slow cooling is less than Because the ILYS of steel 3 at a slower cooling rate of 12 °C per minute is lower than the cooling rate of the filter (Figure 14 and Table 19). ITT steel 4 states that the slow cooling rate of 120 C per minute is lower than the rapid cooling rate (Table 18).
Figures 7 and 14 show that as the cooling rate decreases, the ferrite grain size increases and iIYS decreases. The detrimental effect of the coarse ferrite grain size is overcome by the combined effect of lower soluble nitrogen and lower YS, resulting in a lower ITT at this low cooling rate of 120°C per minute. The much lower ITT of steel than chair I in this, rapid cooling may be due to the less free nitrogen in the presence of aluminum.
At a slower cooling rate of 12 °C per minute, steel S shows the lowest ITT than steels 2, 3 and 4. The ferrite grains of steel 5 at this cooling rate are coarser and the increase in yield strength is much smaller than those of steel 5. 2-4. The much lower ITT is therefore clearly due to the presence of aluminum, which removes soluble nitrogen and causes little or no increase in yield strength than AIN.
TEMPERATURE "C
120 C/min
0---'0 120 °(1 min
TEMPERATURE O(
COOLING RATE