Research Article
Immobilization of acetylcholinesterase
in nanofibrous PVA/BSA membranes by
electrospinning
Electrospinning, a simple and versatile method to fabricate nanofibrous supports,
has attracted continuous attention in the field of enzyme immobilization. In this
study, acetylcholinesterase (AChE) has been successfully immobilized in PVA
nanofibers
via
electrospinning of a mixture of AChE, BSA as an enzyme stabilizing
additive and PVA. The maximum activity recovery of immobilized AChE was
about 40%. In comparison with free enzyme, the immobilized AChE showed
improved stability while retaining a considerable amount of activity at lower pH
values. Moreover, the immobilized AChE retained
434% of its initial activity
when stored at 30
1
C for 100 days and retained 70% of its initial activity after ten
consecutive reactor batch cycles.
Keywords:Acetylcholinesterase / Electrospinning / Immobilization / Nanofiber / PVA
Received:January 6, 2009;revised:October 21, 2009;accepted:October 27, 2009 DOI:10.1002/elsc.200900001
1
Introduction
Enzyme immobilization has been a popular strategy for most large-scale applications due to the ease in catalyst recycling, continuous operation, and product purification [1, 2]. The most explored approach to immobilize enzymes has involved
the use of solid supportsviaa variety of mechanisms, such as physical adsorption, covalent bonds, or entrapment/encapsu-lation [3]. With increasing demand for nanotechnology, elec-trospinning has become a novel technique for generating composite nanofibers. Electrospinning is a method for producing nanofibers from a variety of materials with fiber diameters ranging from several micrometers down to tens of nanometers [4–6]. The electrospun nanofibrous membrane has high specific area and porous structure, so there are
Atousa Moradzadegan1
Seyed-Omid Ranaei-Siadat1
Azadeh Ebrahim-Habibi2
Mohammad Barshan-Tashnizi1
Rouhollah Jalili3
Seyed-Fakhraddin Torabi4 Khosro Khajeh5
1NanoBiotechnology
Engineering Lab., Department of
Biotechnology, Faculty of Energy Engineering and New Technologies, Shahid Beheshti University, GC, Tehran, Iran
2Endocrinology and
Metabolism Research Center, Tehran University of Medical Sciences, Dr. Shariati Hospital, Tehran, Iran
3New Ideas Research
Institute (NIRI), Tehran, Iran
4Department of
Biotechnology, University of Tehran, Tehran, Iran
5Department of
Biochemistry, Faculty of Biological Science, Tarbiat Modares University, Tehran, Iran
Current Address: Department of Biology, Sciences and Research Branch,
Islamic Azad University, Tehran, Iran
Additional corresponding author: Dr. Seyed-Omid Ranaei-Siadat
E-mail: O_ranaei@sbu.ac.ir Abbreviations: AChE, acetylcholinesterase; ATChI,
acetylthio-cholineiodide; GTA,glutaraldehyde
excellent candidates for filtration, drug delivery carrier, tissue engineering, wound dressing, nano-sensors, and enzyme immobilization [7–9]. Immobilization of enzymes on both natural and synthetic polymeric supports has been widely reported [10–17]. PVA can be used for enzyme immobilization by electrospinning since it has good properties in forming membranes and fibers. Besides, it is a biologically compatible, nontoxic, hydrophilic, and readily available low-cost polymer [18–22]. Cellulase loaded at 10% in 200 nm diameter PVA fibers exhibited 65% of original activity [23]. Immobilization of lipase in 100–150 nm diameter PVA nanofibrous, enzyme loading in these biocomposite fibers reached as high as 50% [24]. Glucose oxidase loaded at 8% in 70–250 nm diameter PVA fibers and chronoamperometric measurmens demon-strated that electrospun fibrous enzymatic electrodes exhibited a rapid response (1 s) and a higher response current (mA level) to glucose in the normal and diabetic level [25] Acet-ylcholinesterase (AChE) (E.C 3.1.1.7) is a serine hydrolase, which catalyzes the hydrolysis of the neurotransmitter acet-ylcholine [26]. Carbamates and organophosphates are potent inhibitors of AChE, which causes the blocking of the nerve signal transference into the postsynaptic membrane [27]. Because of the uniform mechanism of signal transduction in animals, these inhibitors have been used as agricultural pesti-cides as well as chemical warfare agents (nerve agents) [28]. Immobilized AChE can be used for construction of biosensor for pesticide determination [29, 30].
Two main methods have been employed to make an assemblage of enzymes and polymeric fibers via electrospin-ning: (i) immobilization of the enzyme on the outer surface of the nanofibers and (ii) mixing enzyme with the polymer solution and subsequently spinning. In the first approach, polymeric materials can be used, which are soluble in organic media. However, enzyme is immobilized only on the outer surface of the fibers while the interior of the fibers remains inactive. In contrast to the first approach, water-soluble polymers such as PVA or dextran can be used as a solid support in the second approach. However, dissolution of the fibers in an aqueous environment and subsequent enzyme leaching would be a problem. To overcome this problem, it was suggested to use vapor glutaraldehyde (GTA) [24] so that the nanofibers can be cross-linked to prevent support dissolution. In the present study, AChE was immobilized using mixed spinning approach of the enzyme, PVA, and BSA biocomposite nanofibers with the use of vapor GTA. Kinetic parameters of both free and immobilized enzymes were determined. Also storage stability of the immobilized system was compared with free enzyme and reusable stability of immobilized enzyme was assayed.
2
Materials and methods
2.1 Materials
AChE (E.C 3.1.1.7) was expressed with the baculovirus system [31]. GTA 25% was purchased from TAAB Laboratories (England, UK). BSA, acetylthiocholineiodide (ATChI) and 5,50 -dithiobis-(2-nitrobenzoic acid) were obtained from Sigma-Aldrich (MO,
USA). PVA 72000 and498% of degree of hydrolysis and all other agents used in the experiments were of analytical grade and were from Merck (Darmstadt, Germany). Aqueous solutions were prepared in doubly distilled deionized water.
2.2 Methods
2.2.1 Preparation of nanofibrous membranes
PVA aqueous solution (6% w/w) was prepared by dissolving PVA powder in deionized water at 801C with gentle stirring for 4 h to form homogenous solution after removing air bubbles. After the solution was cooled to the room temperature, prepared solutions in different concentrations were placed in a plastic syringe (1 ml) bearing a metal capillary (0.8 mm diameter) connected to a high-voltage power supply; the grounded counter electrode connected to the aluminum foil collector. Electrospinning was performed at 10–12 kV voltage, 15 cm distance between the needle tip and the collector. The flow rate of the solution was controlled by a syringe pump to maintain at 26 mL/h from the needle outlet. It usually takes 45 min to obtain a sufficiently thick membrane that can be detached from the aluminum foil collector.
PVA/BSA solution was prepared by gently mixing PVA and BSA solution to yield the spinning solution with final concentrations of 6% w/w and 10 mg/mL for PVA and BSA in spinning solution, respectively. The same procedure was used to prepare PVA/BSA/AChE solution by adding an aqueous solution of AChE to 25 mM phosphate buffer, pH 7.4, different ratios of BSA and AChE to PVA were used. An aliquot of 1 mL of each solution under the mentioned conditions was electrospun to form the biocomposite nanofibrous membranes. The electrospinning was performed at room temperature and the resulting PVA/BSA and PVA/BSA/AChE biocomposite fibers were collected on the surface of aluminum foil collector.
Covalent attachment of BSA in nanofibrous PVA
membranes was carried out by cross-linking the electrospun PVA/BSA membranes at room temperature by GTA vapor for 12 h. PVA/BSA membrane (20 mg) was immersed in glass vials containing deionized water at room temperature and was agitated at 300 rpm for 120 min to detach all un-bounded protein from the membrane. The amount of released protein was determined by measuring the residual protein present in the supernatants by Bradford method using coomassie brilliant blue G-250 and BSA as the standard protein [32]. The amount of immobilized protein was estimated by subtracting the amount of protein in the supernatants from the total amount of protein used in the immobilization procedure. The protein loading efficiency was determined by dividing the amount of immobilized BSA (mg) in the membrane to the whole support membrane mass (g).
and 3mg/mL AChE was electrospun into biocomposite nano-fibrous membranes and used for determination of kinetic parameters, pH profile, and stability experiments. The morphology of this nanofibrous PVA/BSA/AChE membrane was characterized by a Philips XL-30 scanning electronic microscope after being sputtered with gold.
2.2.2 Catalytic activity determination
The AChE activity was determined according to the ellman method [33, 34] using ATChI as substrate and 5,50 -dithiobis-(2-nitrobenzoic acid) as chromogen and increasing absorbance at 412 nm was measured for 1 min. For the immobilized enzyme assay, the electrospun nanofibrous membrane was removed from the collector and cut into 1 cm1 cm pieces,
3 mg of nanofibers containing immobilized enzymes were added to each mixture reaction (1 mL) in vials, vortexed for 1 min, and then centrifuged rapidly at 16 000g for 1 min. The product concentration that was proportional to the hydrolyzed ATChI, therefore, to the intensity of the yellow solution in the resulting supernatant was determined spec-trophotometrically at 412 nm. One unit of enzymatic activity was defined as the amount of enzyme that catalyzes 1mmol of substrate to productperminute. Specific activity was expressed as unitspermilligram of protein in the assay medium (1 mL) and the relative activity was defined as the ratio between the specific activity of a bound enzyme and free enzyme. All reactions and measurements were carried out at room temperature. In order to determine kinetic parameters (Km andVmax) for free and immobilized enzyme, several concen-trations of ATChI were used ranging from 0.01 to 1 mM. The activity of free and immobilized enzyme was determined within the pH range of 4 to 8.5.
The amount of protein in the different samples was estimated according to the Bradford method using BSA as standard [34].
2.2.3 Storage stability and reusability assay
Activities of the free and immobilized AChE were determined after storage in 25 mM phosphate buffer solution (pH 7.4) at 4 and 301C. The measurements were performed at intervals of 2 wk within a period of 100 days. The reusability of bound AChE was examined by conducting the activity measurement of bound AChE at time intervals of 15 min. After each activity measurement, the bound AChE was washed three times with 25 mM phosphate buffer. The supports were then centrifuged, the supernatant was decanted, and the recycled supports subjected to the activity assay for the second cycle and so on.
3
Results and discussion
3.1 Morphology of PVA/BSA/AChE electrospinning composite membrane
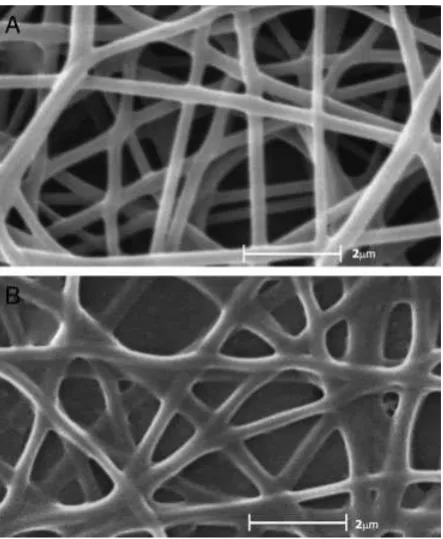
The physical morphology of the electrospun samples was examined using the scanning electron microscope. PVA
solu-tion can be electrospun into nanofibers with diameter around 190 nm as shown in Fig. 1A. With the addition of BSA and AChE, electrospun fibers became irregular but the diameter of the fibers did not change (Fig.1B). As a protein, it is impossible for BSA or AChE to be electrospun alone into nanofibers due to its complicated three-dimentional structures as well as strong inter- and intra-molecular forces [12]. The blending of BSA and AChE with PVA can interrupt its complex struc-ture. PVA has a capacity for secondary binding; because of this, the mixture of PVA and the enzyme can be electrospun and form nanofibers by electrospinning. On the other hand, the interaction of PVA and protein resulted in the reduction of stability of PVA solution and induced the appearance of irregular [24].
3.2 Covalent immobilization of BSA in PVA membrane
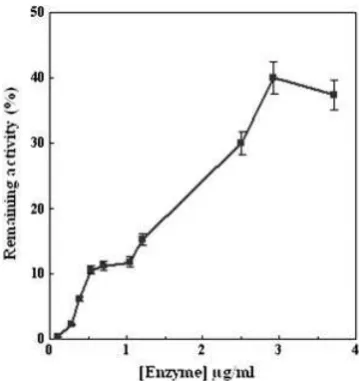
After co-spinning of different concentration of BSA with PVA, the nanofibers were incubated with vaporized GTA for 12 h. The amount of released BSA was measured (Fig. 2). A signif-icant decrease was observed after increasing the concentration of BSA from 3 to 18 mg/mL. The optimum BSA concentration for immobilization of AChE in nanofibers was found to be 10 mg/mL. GTA acts as a linker and links amine groups of lysine residues of BSA and due to the conglutination between BSA molecules, creates a protein networks onto PVA membranes. This may be the reason for more immobilization when high concentration of BSA was used.
3.3 Enzyme immobilization
AChE was covalently immobilized in modified membranes using vapor GTA. Because of low concentration of AChE with respect to BSA, no activity was detected in the supernatant at the end of immobilization procedure. According to the
DrosophilaAChE crystal structure [35] there are more than 20 lysine residues available on the AChE surface for reaction with GTA.
GTA is highly reactive toward both the amine and hydroxyl groups and acts as a linker [36]. These mechanisms result in covalent attachment of AChE in PVA/BSA membranes. The activity of immobilized AChE with different enzyme loading (0.2–3.5mg/mL) was determined (Fig. 3). The relative immo-bilized AChE activity (activity recovery) was more than 40%. Decrease in activity is usually observed after enzyme immo-bilization. Enzyme immobilization via covalent binding will change enzyme conformation or reduce its flexibility and may cause reduction in enzyme mobility to induce fit to a substrate [37] that may lead to a reduction of AChE activities. Moreover, GTA as a cross-linking agent reacts with hydroxyl groups from PVA chains and causes the PVA membranes to pack more densely, thus decreasing surface area of the support as well as mass transfer through it [23]. Besides, cross-linking can reduce membrane porosity, which in turn causes limited accessibility of substrates to active site.
In addition, different concentrations of BSA (0, 3, 6, 10, 14,
and 18 mg/mL) were electrospun into biocomposite
membranes containing 6% w/w PVA and 3mg/mL AChE. All samples were subjected to cross-linking with vapor GTA as mentioned in Section 2. The fibers were washed three times thoroughly with phosphate buffer solution (25 mM, pH 7.4) to extract out un-bonded enzymes. Buffer solutions were found to have no detectable enzyme activity, indicating that there was negligible enzyme leakage during washing procedure. There-fore, the yield of AChE immobilization was found to be about 100%. The activity of the resulting immobilized AChE was compared with the free enzyme to determine enzyme activity
retention after immobilization. Results showed that the presence of BSA had a significant effect on AChE activity retention. In comparison to PVA, a significant increase in the retaining activity of immobilized enzyme (23–40%) was observed using PVA and BSA (10 mg/mL), simultaneously. Several speculate could be used to explain this phenomenon. First, the BSA-modified fibers could create a biocompatible microenvironment for the immobilized AChE, which usually leads to high activity retention of the immobilized enzyme [10]. Second, decreased number of covalent bonds with the support (number of hydroxyl groups of PVA is very high compared with NH2 groups of BSA) may cause improvement in the catalytic efficiency of the immobilized enzyme. Finally, using BSA as a linker between AChE and the support would be in favor of enzyme flexibility.
Although the presence of BSA led to a significant increase in the activity retention of immobilized AChE, increasing loaded protein from 14 to 18% resulted in decreasing activity reten-tion from 40 to 33%. This may be due to the presence of large numbers of amino groups on the surface of BSA molecules, which are potential reaction sites for covalent coupling with enzyme. Thus, it becomes easier to immobilize one enzyme molecule through multipoint covalent attachments on the BSA-modified membrane with higher concentrations of BSA, which negatively affect the enzymatic activity. Based on observed results, it has been suggested that enzyme immobi-lization using 3mg/mL AChE into biocomposite membrane loaded with 10% BSA would be the condition to achieve maximum activity retention.
3.4 pH-activity profile
The pH is one of the important parameters capable of altering enzymatic activities in aqueous solution. The effect of pH on Figure 2.Immobilization of several concentration of BSA onto
PVA membranes. For more details please see Section 2. Figure 3.remaining activity of the immobilized enzyme. All experimentsEffect of several concentrations of enzyme on the
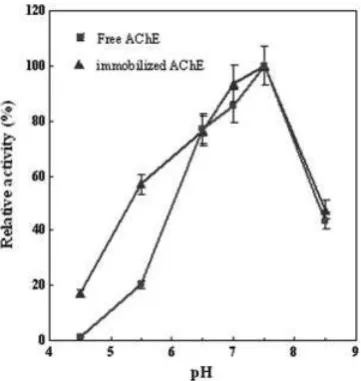
the activity of the free and immobilized AChE was investigated within range of pH 4.5–8.5 at room temperature. Relative activity as a function of pH is depicted in Fig. 4. The optimum pH of immobilized AChE was found to be about 7.5. So, the three dimensional structure of enzyme’s active site may not be affected from immobilization procedure and optimum pH of immobilized enzyme can be observed as free enzyme [38]. The immobilized enzyme was less sensitive to pH changes at acidic pHs than alkaline pHs compared with that of free AChE. These data indicate that the pH stability of AChE could be enhanced by the immobilization process at low pH values [39].
PVA/BSA/AChE biocomposite contained about 15% w/w protein and 85% polymer. The isoelectric point of BSA is 4.8 and is positively charged at lower pH values [40]. The presence of protonated BSA amino groups on the surface of the membrane might repel protons from the region in the vicinity of the surface and create a higher pH at the boundary layer between the support and the bulk solution. This micro-environment with a pH slightly higher than bulk solution may lead to an increase in pH stability of the immobilized enzyme.
3.5 Kinetic parameters
The kinetic parameters of the free and immobilized AChE were determined using acetylthiocholine iodide as substrate at constant temperature and pH. TheKmandkcatof the immo-bilized enzyme were calculated from Lineweaver–Burk plot and the results were compared with those obtained for the free AChE (Fig. 5). In comparison with the free AChE, the kcat obtained for immobilized AChE (3500 sec 1) was approxi-mately four times lower as shown in Table 1. TheKmvalues were estimated as 0.5 and 0.3 mM for immobilized and free
enzyme, respectively. There was approximately a 1.7-fold increase inKmvalue for immobilized enzyme. This alteration was either due to the conformational changes of the enzyme resulting in a lower possibility of forming a substrate–enzyme complex or the lower accessibility of the substrate to the active sites of the immobilized enzyme caused by the increased diffusion limitation.
3.6 Storage and reuse stability
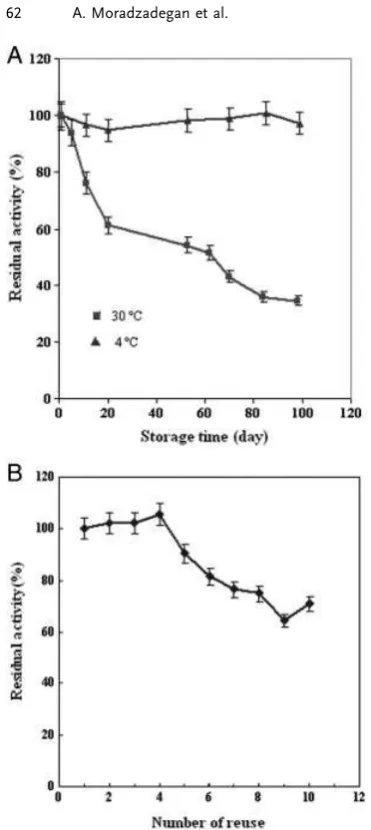
In order to investigate the industrial practicability of an immobilized enzyme, the loss of enzyme activity, known as storage stability, is an important parameter to be taken into account. The immobilized AChE was stored in phos-phate buffer solution at 4 and 301C separately and activities were measured periodically over duration of 100 days. Upon 100 days of storage, the catalytic activity of immobilized enzyme was retained 490% at 41C and 434% at 301C (Fig. 6A).
Figure 4.Effect of pH on the activity of free and immobilized AChE. The reactions were carried out at room temperature under the variety of pH. A solution of 6% w/w PVA, 10 mg/mL BSA, and 3mg/mL AChE was electrospun into biocomposite
nanofi-brous membranes and used as immobilized enzyme. For more details see Section 2.
Figure 5. The Michaelis–Menten diagram of soluble and
immobilized form of AChE. Soluble and immobilized enzymes. Insets: Lineweaver-Burk plots of soluble and immobilized. A solution of 6% w/w PVA, 10 mg/mL BSA, and 3mg/mL AChE was
electrospun into biocomposite nanofibrous membranes and used as immobilized enzyme. For more details see Section 2.
Table 1.Activity recovery and kinetic parameters of soluble and immobilized AChE
Enzyme Km(mM) Vmax(mmol/min mL) kcat(s 1) R(%)a)
Soluble AChE 0.3 0.37 15 000 –
Immobilized AChEb)
0.5 0.27 3500 40%
a)Ris the remaining activity of the immobilized enzyme.
In addition, reusability of immobilized enzymes that was important for their practical application was carried out by measuring the activity of the immobilized enzyme successive times. As shown in Fig. 6B, the immobilized enzyme main-tained more than 70% of its original activity after ten reuses, which is better than previous study that AChE was immobi-lized on PANCHI-B membranes, and the enzyme activity loss was less than 50% after ten times [41]. The loss of catalytic activity may be explained by the following two reasons. First, there may be some AChE molecules, which were not chemi-cally bonded on PVA fibers but only physichemi-cally embedded, were lost during the process of measurement. Second, the diameter of PVA fibers may become larger and the surface area
got smaller after several arrays due to the hydrophilicity of PVA [23].
4
Concluding remarks
PVA/BSA/AChE biocomposite nanofibrous membrane could be fabricated by electrospinning and the enzyme molecules could be covalently bound to the nanofiber with GTA. With the huge specific surface area provided by the nanofiber, immobilized AChE retains 40% of its initial activity. After immobilization, there was a 1.7-fold increase inKmvalue for immobilized enzyme and in addition, immobilized AChE can retain most of the activity at lower pH values when compared with the free enzyme. Immobilized system had good storage stability and it was shown to have better reusable stability than previous studies. This immobilized system may have industrial application for pesticide determination.
Acknowledgements
The authors express their gratitude to Research Center of Basic Science, Shahed University for the financial support during the course of this project.
Conflict of interest statement
The authors have declared no conflict of interest.
References
[1] Li, S. F., Chenb, J. P., Wu, W. T., Electrospun polyacrylonitrile nanofibrous membranes for lipase immobilization. J. Mol. Catal. B Enzym.2007,47, 117–127.
[2] Mozhaev, V. V., Melik-Nubarov, N. S., Sergeeva, M. V., Siksnis, V., Martinek, K., strategy for stabilizing enzymes part one: increasing stability of enzymes via their multi-point interaction with a support. Biocatal. Biotransfor. 1990, 3, 179–187.
[3] Wingard, L. B., Jr., Katchalski-Katzir, E., Goldstein, L., (Eds.), Immobilized enzyme principles, in:Applied Biochemistry and Bioengineering, Academic Press, New York1976, p. 364. [4] Reneker, D. H., Chun, I., Nanometre diameter fibres of
polymer, produced by electrospinning.Nanotechnology1996,
7, 216–223.
[5] Demir, M. M., Yilgor, I., Yilgor, E., Erman, B., Electrospin-ning of polyurethane fibers.Polymer2002,43, 3303–3309. [6] Li, D., Xia, Y., Electrospinning of nanofibers: reinventing the
wheel?Adv. Mater.2004,16, 1151–1170.
[7] Huang, Z. M., Zhang, Y. Z., Kotaki, M., Ramakrishna, S., A review on polymer nanofibers by electrospinning and their applications in nanocomposites.Compos. Sci. Technol.2003,
63, 2223–2253.
[8] Liang, D., Hsiao, B. S., Chu, B., Functional electrospun nanofibrous scaffolds for biomedical applications.Adv. Drug Deliver. Rev.2007,59, 1392–1412.
Figure 6. (A) Storage stability of immobilized AChE in 4 and 301C. All experiments were carried out in phosphate buffer (pH 7.4). (B) The influence of the number of reuse on the activity of immobilized AChE with repeated cycles. All cycles were carried out at room temperature. A solution of 6% w/w PVA, 10 mg/mL BSA, and 3mg/ml AChE was electrospun into biocomposite
[9] Lannutti, J., Reneker, D., Ma, T., Tomasko, D., Farson, D., Electrospinning for tissue engineering scaffolds, Mater. Sci. Eng. C.2006,27, 504–509.
[10] Ye, P., Xu, Z. K., Che, A. F., Wu, J., Seta, P., Chitosan-tethered poly(acrylonitrile-co-maleic acid) hollow fiber membrane for lipase immobilization. Biomaterials 2005, 26, 6394–6403.
[11] Huang, X. J., Xu, Z. K., Wan, L. S., Innocent, C., Seta, P., Electrospun nanofibers modified with phospholipid moieties for enzyme immobilization. Macromol. Rapid Commun.
2006,27, 1341–1345.
[12] Xie, J., Hsieh, Y. L., Ultra-high surface fibrous membranes from electrospinning of natural proteins: casein and lipase enzyme.J. Mater. Sci.2003,38, 2125–2133.
[13] Liu, Z. M., Xu, Z. K., Wang, J. Q., Yang, Q., Wu, J., Seta, P., Surface modification of microporous polypropylene membranes by the grafting of poly(c-stearyl-L-glutamate).
Eur. Polym. J.2003,39, 2291–2299.
[14] Ye, P., Xu, Z. K., Wu, J., Innocent, C., Seta, P., Nanofibrous poly(acrylonitrile-co-maleic acid) membranes functionalized with gelatin and chitosan for lipase immobilization. Bioma-terials2006,27, 4169–4176.
[15] Ye, P., Xu, Z. K., Wang, Z. G., Wu, J., Denga, H. T., Seta, P., Comparison of hydrolytic activities in aqueous and organic media for lipases immobilized on poly(acrylonitrile-co-maleic acid) ultrafiltration hollow fiber membrane. J. Mol. Catal. B Enzym.2005,32, 115–121.
[16] Ye, P., Xu, Z. K., Wu, J., Innocent, C., Seta, P., Entrusting poly(acrylonitrile-co-maleic acid) ultrafiltration hollow fiber membranes with biomimetic surfaces for lipase immobilization. J. Mol. Catal. B Enzym. 2006, 40, 30–37.
[17] Amounas, M., Innocent, C., Cosnier, S., Seta, P., A membrane based reactor with an enzyme immobilized by an avidin–biotin molecular recognition in a polymer matrix.
J. Membrane Sci.2000,176, 169–176.
[18] Ding, B., Kim, H. Y., Lee, S. C., Lee, D. R., Choi, K. J., Preparation and characterization of nanoscale poly(vinyl alcohol) fibers via electrospinning. Fiber Polym. 2002, 3, 73–79.
[19] Lozinsky, V. I., Plieva, F. M., Poly (vinyl alcohol) cryogels employedas matrices for cell immobilization. 3. Overview of recent research and developments.Enzyme Microb. Technol.
1998,23, 227–242.
[20] Zeng, J., Aigner, A., Czubayko, F., Kissel, T., Wendorff, J. H., Greiner, A., Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules
2005,6, 1484–1488.
[21] Koski, A., Yim, K., Shivkumar, K., Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett.
2004,58, 493–497.
[22] Djennad, M., Benachour, D., Berger, H., Schomacker, R.,. Poly (vinyl alcohol) ultrafiltration membranes: synthesis, characterization, the use for enzyme immobilization. Eng. Life Sci.2003,3, 446–452.
[23] Wu, L., Yuan, X., Sheng, J., Immobilization of cellulase in nanofibrous PVA membranes by electrospinning.
J. Membrane Sci.2005,250, 167–173.
[24] Ren, G., Xu, X., Liu, Q., Cheng, J., Yuan, X., Wu, L., Wan, Y., Electrospun poly(vinyl alcohol)/glucose oxidase biocompo-site membranes for biosensor applications. React. Funct. Polym.2006,66, 1559–1564.
[25] Wang, Y., Hsieh, Y. L., Immobilization of lipase enzyme in polyvinyl alcohol (PVA) nanofibrous membranes.
J. Membrane Sci.2008,309, 73–81.
[26] Ranaei-Siadat, S. O., Lougarre, A., Lamouroux, L., Ladur-antie, C., Fournier, D., The effect of engineered disulfide bonds on the stability of Drosophila melanogaster acet-ylcholinesterase.BMC Biochem.2006,7, 12–18.
[27] Pogacnik, L., Franko, M., Determination of organophosphate and carbamate pesticides in spiked samples of tap water and fruit juices by a biosensor with photothermal detection.
Biosens. Bioelectron.1999,14, 569–578.
[28] Singh, A. K., Flounders, A. W., Volponi, J. V., Ashley, C. S., Wally, K., Schoeniger, J. S., Development of sensors for direct detection of organophosphates. Part I: immobilization, characterization and stabilization of acetylcholinesterase and organophosphate hydrolase on silica supports. Biosens. Bioelectron.1999,14, 703–713.
[29] Amine, A., Mohammadi, H., Bourais, I., Palleschi, G., Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens. Bioelectron. 2006, 21, 1405–1423.
[30] Cremisini, C., Di Sario, S., Mela, J., Pilloton, R., Palleschi, G., Binding of acetylcholinesterase to multiwall carbon nano-tube-cross-linked chitosan composite for flow-injection amperometric detection of an organophosphorous insecti-cide.Anal. Chim. Acta1995,311, 273–280.
[31] Chaabihi, H., Fournier, D., Fedon, Y., Bossy, J. P., Ravallec, M., Devauchelle, G., Cerutti, M., Biochemical characteriza-tion of Drosophila melanogaster acetylcholinesterase expressed by recombinant baculoviruses.Biochem. Biophys. Res. Commun.1994,203, 734–742.
[32] Bradford, M. M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal. Biochem. 1976, 76, 248–254.
[33] Ellman, G. L., Courtney, K. D., Andres, V., Feather-StoneJr, R. M., A new and rapid. colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95.
[34] Clark, D. S., Prospects for exploiting immobilization to modify enzyme activity.Trends Biotechnol.1994,12, 439–445. [35] Godeau, F., Saucier, C., Kourilsky, P., Replication
inhibi-tion by nucleoside analogues of a recombinant Auto-grapha californica multicapsid nuclear polyhedrosis virus harboring the herpes thymidine kinase gene driven by the IE-1(0) promoter: a new way to select recombinant baculo-viruses.Nucleic Acids Res.1992,20, 6239–6246.
[36] Araujo, A. M., NevesJr, M. T, Azevedo, W. M., Oliveira, G. G., FerreiraJr, D. L., Coelho, R. A. L., Figueiredo, E. A. P., CarvalhoJr, L. B., Polyvinyl alcohol-glutaraldehyde network as a support for protein immobilisation.Biotechnol. Techni-ques1997,11, 67–70.
Activity, stability and co-immobilization.J. Biotechnol.2007,
131, 111–120.
[38] Sahin, F., Demirel, G., Tumt. urk, H., A novel matrix for the. immobilization of acetylcholinesterase.Int. J. Biol. Macromol.
2005,37, 148–153.
[39] Yabushita, I., Studies on the properties of immobilized urokinase: effects of pH and temperature.Biotechnol. Appl. Biochem.1988,10, 294–300.
[40] Su, T. J., Lu, J. R., Thomas, R. K., Cui, Z. F., Effect of pH on the Adsorption of Bovine Serum Albumin at the Silica/Water Interface. J. Phys. Chem. B 1999, 103, 3727–3736.