Burkholderia
sp. AS ANTIFUNGAL-PRODUCING BACTERIA
TO SUPPRESS
Ganoderma boninense
IN OIL PALM
RIKA FITHRI NURANI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
CERTIFICATE OF ORIGINALITY
I hereby declare that this Master thesis entitled Burkholderia sp. as Antifungal-Producing Bacteria to Suppress Ganoderma boninense in Oil Palm is the result of my own work under supervisor committee and has not been submitted in any forms to any colleges. Sources of information derived or quoted are from published and unpublished works of other authors which has been mentioned in the text and listed in the reference chapter at the end of this thesis.
Bogor, August 2014
Rika Fithri Nurani
RINGKASAN
RIKA FITHRI NURANI. Burkholderia sp Sebagai Bakteri Penghasil Senyawa Antifungi dalam Menekan Pertumbuhan Ganoderma boninense pada Kelapa Sawit. Dibimbing oleh ARIS TRI WAHYUDI dan NURITA TORUAN-MATHIUS
Indonesia merupakan salah satu produsen minyak kelapa sawit di dunia, sehingga kelapa sawit merupakan salah satu komoditas penting di Indonesia. Indonesia menyediakan 46% minyak kelapa sawit di dunia dan permintaan akan minyak kelapa sawit semakin lama semakin meningkat. Penyakit yang paling banyak ditemukan di perkebunan kelapa sawit adalah busuk pangkal batang yang disebabkan oleh G. boninense. Berbagai usaha telah dilakukan untuk mencegah penyebaran penyakit ini, namun belum menunjukkan hasil yang optimum.
Burkholderia sp. yang diisolasi dari rizosfer dan dalam jaringan (endofit) yang berasal dari tanaman kelapa sawit yang sehat menunjukkan potensi dalam menekan pertumbuhan G. boninense secara in vitro. Burkholderia sp. telah dilaporkan sebagai agen biokontrol karena kemampuannya dalam memproduksi beberapa senyawa antifungi. Tujuan penelitian ini adalah untuk mengisolasi
Burkholderia sp indigenus yang berpotensi dalam menghambat pertumbuhan G. boninense secara in vitro dan in vivo, dan profil komunitas bakteri di dalam tanaman setelah diberikan perlakuan Burkholderia sp. dan G. boninense.
Lima isolat Burkholderia sp. rizosfer (B313, B51a, B52c, B51b, B52a) dan satu isolat Burkholderia sp. endofit (B212) telah berhasil diisolasi dari perkebunan kelapa sawit. Isolat Burkholderia sp. kemudian digunakan dalam uji antagonis terhadap pertumbuhan G. boninense di dalam media PDA.
Burkholderia sp. dengan aktivitas antagonis yang tertinggi kemudian digunakan untuk uji in vivo. Profil komunitas bakteri endofit setelah perlakuan Burkholderia
sp. dan G. boninense dianalisis dengan menggunakan DGGE (Denaturing Gradient Gel Electrophoresis). Selanjutnya gen yang diduga terkait dengan biosistesis senyawa antifungi dari Burkholderia sp. dikonfirmasi dengan menggunakan primer spesifik pyrrolnitrin (prn), pyoluteorin (plt), phenazine (phz), dan DAPG (phl).
Burkholderia sp. dengan kode B212 menunjukkan aktivitas hambat yang paling tinggi yaitu dengan nilai PIRG 24,38%. Genom dari isolat B212 tersebut menunjukkan hasil PCR dengan ukuran 790 bp menggunakan primer prn. Hasil sekuens menunjukkan bahwa hasil PCR tersebut 99% identik dengan gen prnD
parsial dari B. cepacia strain ESR63. Hasil uji in vivo konsorsium B212 dan B52a pada tanaman yang tidak diberikan infeksi G. boninense menunjukkan adanya pertumbuhan tanaman yang lebih baik dibandingkan dengan kontrol. Namun, konsorsium B212 dan B52a tidak menurunkan tingkat infeksi dari G.boninense
Burkholderia sp. memiliki potensi sebagai agen biokontrol untuk fungi patogen seperti G. boninense, namun metode aplikasinya belum tepat. Aplikasi
Burkholderia sp dan G. boninense pada kelapa sawit secara bersamaan
memberikan hasil yang belum memuaskan. Metode aplikasi Burkholderia sp. endofit sebagai biokontrol terhadap G. boninense perlu dioptimasi dan riset yang lebih mendalam. Dari hasil DGGE menunjukkan bahwa introduksi bakteri dari luar dapat mengubah komunitas bakteri yang ada di dalam jaringan tanaman. Kata kunci : gen penyandi antibiotik, busuk pangkal batang, agen biokontrol, bakteri endofit, aplikasi perendaman.
SUMMARY
RIKA FITHRI NURANI. Burkholderia sp. as Antifungal-Producing Bacteria to Suppress Ganoderma boninense in Oil Palm. Supervised by ARIS TRI WAHYUDI and NURITA TORUAN-MATHIUS.
Indonesia is one of the world palm oil producer, which make oil palm is one of the most important comodity in Indonesia. Indonesia provide 46% of palm oil in the world and the demand is increase continously. Though in fact, the land for oil palm plantation has been limited. Basal Stem Rot (BSR) disease caused by
G. boninense is one of the most serious diseases in oil palm. Many attempts have
been done to prevent or reduce infection of this disease, but they have not provided optimum results. Burkholderia sp. isolated from rhizosphere and root tissue of symptomless oil palm showed potentials in suppressing G. boninense
growth in vitro. Burkholderia sp. has been reported as a biocontrol agent regards to its ability to produce vary of antifungal compounds. The objective of this research were to isolate the Burkholderia sp. potential to suppress G. boninense
growth in vitro and in vivo, and community profile of endophyte bacteria after treated with Burkholderia sp. and G. boninense.
There were five isolates of rhizosphere Burkholderia sp. (B313, B51a, B52c, B51b, B52a) and one endophyte Burkholderia sp. (B212) isolated from oil palm plantation. The Burkholderia sp. isolates were used in antagonist test against
G. boninense growth on PDA media. Burkholderia sp. with the highest antagonist
activity were applied to oil palm germinated seed for in vivo test. Endophyte bacteria community profile was analyzed by using Denaturing Gradient Gel Electrophoresis (DGGE) after treated with Burkholderia sp. and G. boninense. Then, antifungal biosynthesis related gene from Burkholderia sp. was confirmed by using PCR with pyrrolnitrin (prn), pyoluteorin (plt), phenazine (phz), and DAPG (phl) primers.
Endophyte Burkholderia B212 showed the highest antagonist activity against G. boninense growth in vitro with PIRG 34.38%. B212 genome was yield an expected PCR product by using prn primers (790-bp). Sequence BLAST result showed the gene was 99% identical with B. cepacia partial prnD gene, strain ESR63. In vivo test of B212 showed that treatment of Burkholderia B212 on plant without G. boninense infection increased the height and biomass of the plant. However, B212 did not decrease the disease severity on G. boninense infected plant. In addition it suppressed the plant height and biomass compare to control plant. DGGE analysis was show that community profile of bacteria endophyte on plant treated with Burkholderia sp. was different with those in untreated plants. However, endophyte bacteria community profile in plants applied with G. boninense was similar with those in control plants (without G. boninense).
Burkholderia sp. has a potential as a biocontrol agent for pathogen such as
already exist in plants tissue. observation of Burkholderia and G. boninense
interaction in oil palm should be conduct longer.
© Copy Right IPB, 2014
All Rights Reserved by Law
No part or all of this work may be reproduced without citing the source. Copying may be done only on the basis of education, research, scientific writting, reports or reviews; and which should not cause any prejudice to IPB.
Thesis
submitted in partial fulfilments for the degree Master Science
in
Microbiology Study Program
Burkholderia
sp. AS ANTIFUNGAL-PRODUCING BACTERIA
TO SUPPRESS
Ganoderma boninense
IN OIL PALM
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2014
Title : Burkholderia sp. as Antifungal-Producing Bacteria to Suppress
Ganoderma boninense in Oil Palm
Name : Rika Fithri Nurani NIM : G351120311
Approved by Supervising committee
Prof Dr Aris Tri Wahyudi, MS Head
Dr Nurita Toruan-Mathius, MS Member
Endorsed by
Head of Study Program Microbiology
Prof Dr Anja Meryandini, MS
Dean of Graduate School
Dr Ir Dahrul Syah, MScAgr
Date of Examination: 28 August 2014
PREFACE
I would like to express the deepest appreciation to my supervisor Prof. Dr. Aris Tri Wahyudi for the guidance, suggestions and valuable comments throughout the development of this thesis. I would like to thank my co-suppervisor, Dr. Nurita Toruan-Mathous for never ending support, guidance, and encouragement throughout the whole process. My sincere thanks go to Dr. Tony Liwang as Division Head of Plant Production and Biotechnology, PT. SMART Tbk for the permission to continue my study and for the scholar.
I am grateful to my colleagues in IPB Aar, Randi, Nezha, Lekta, Ayun, Dina, Anja, and Asril for their teamwork and companion and also the whole Microbiology Laboratory staff. My sincere thanks also go to my fellow lab mates in PT. SMART Tbk. ibu Elizabeth, Sinthya, Wisnu, Diessa, Dewi, Esti, Hany, Zulfikar and whom I cannot mention one by one for their helps besides their friendship.
Last but not least, I would like to thank my family, especially my father Lalang Buana, my mother Tri Haryati, and my sisters Ratna dan Ririn for their eternal love, encouragement, and support throughout my life. My special thanks also go to Firman for the support and understanding.
Result of this work has been acccepted and reviewed by Asian Journal of Agricultural Research.
Bogor, August 2014
CONTENTS
LIST OF TABLES vi
LIST OF FIGURES vi
LIST OF ATTACHMENTS vi
INTRODUCTION 1
Background 1
Scientific Problem 2
Objective 2
Research Benefit 2
Research Scope 3
LITERATURE REVIEW 3
G. boninense in Oil Palm 3
Basal Stem Rot Disease 4
Endophyte Microbes 4
Potential Endophyte Bacteria 5
DGGE Analysis of Endophyte Community 6
METHOD 7
Research framework 7
Research Time and Place 8
Burkholderia sp. isolation 8
Antagonist in vitro test 8
In vivo test 8
Detection antifungal gene 10
RESULTS AND DISCUSSION 12
Results 12
Discussion 17
CONCLUSION AND SUGESTION 19
Conclusion 19
Sugestion 20
REFERENCES 20
LIST OF TABLES
1 The signs and symptoms of plants were scored on a disease scale 0-4 4 2 Treatments of Burkholderia sp and G. boninense on oil palm seedling, 9 3 Polymerase chain reaction primers and expected amplification products
from genes encoding enzymes involved in the biosynthesis of several
antibiotics. 11
4 Percentage of inhibition ratio from Burkholderia sp against G.
boninense growth in-vitro. 13
5 Disease severity index (DSI) of treated plant with G. boninense. 13 6 The effect of different progeny on root and leaf total number, lenght,
and dry weight after treatment with G. boninense and Burkholderia
sp. 14
7 The effect of dipping treatment of antagonistic bacteria on seedling of oil palm inoculated with G. boninense in pre nursery at 3 months
after planting 14
LIST OF FIGURES
1 Research framework 7
2 In vitro test of Burkholderia sp. against G. boninense growth. R1 as the control and R2 is the radial growth of G. boninense on trial. 12
3 Burkholderia sp. streaked on agar plate. 12
4 a. Plants treatment left to right (A-D). b. Healthy root with no appearance of fungal mycellia (left). Appearance of fungal mycellia
(red arrow). 13
5 PCR amplification of 16S rRNA gene from oil palm roots after
3months. 15
6 DGGE band profile of endophyte bacteria treatment with Burkholderia
and G. boninense (left) and cluster analysis of endophyte bacterial in oil palm root treated with Burkholderia sp and G. boninense with 1D
Pro Phoretrix software (right). 15
7 Phylogenetic tree of excised bands from DGGE. 16
8 PCR product of pyrrolnitrin and pyoluteorin gene amplification. M : marker, 3. prn primer, 4. plt primer, a. B212, b. B313 , c. B51a , d. B52c , e. B51b, f. B52a. Red arrows showed the expected band size. 16 9 Phylogenetic tree of PrnD gene from B212 and B51a. 17
LIST OF ATTACHMENTS
1 List of sequencing results from Burkholderia sp. isolate. 24 2 List of sequencing results from DGGE band slicing. 27
INTRODUCTION
Background
Oil palm is one of the most important agricultural export crops in Indonesia besides rubber, cocoa, coffee, and spices (Stads et al. 2007). Basal stem rot disease has been a serious threat to the oil palm industry in Indonesia because it shortens the productive life of oil palms and causes serious economic loss. The disease is caused by a white-rot fungi G. boninense and in the past few decades has been spreading rapidly, for instance, in North Sumatra, Indonesia, this disease can lead to losses as much as 50% after repeated planting cycles (25 years) (Corley & Tinker 2003).
The use of fungicides for fungal control did not produce significant results yet (Haas & Defago 2005). This may be due to the fact that by the time treatment is applied, the palms may already have the disease. Antifungal-producing bacteria is a promising biocontrol agent (BCA) to overcome this disease. BCA does not necessarily be a cure for the disease but to slowing or even to stop the disease spread by protect the plant or enhance the plant defense. Endophyte BCAs have more value since it can live inside the plant tissue through the plants lifetime.
Endophyte bacteria is bacteria which live inside the plant tissues without causing apparent harm or symptoms to the host (Munif et al. 2013). Endophyte as the internal plant habitat provide several advantages as BCA. It will be less competition with other microorganisms, sufficient supply with the nutrients, less exposure to environmental stress factors, and better translocation of bacterial metabolites throughout the host plant (Hallmann et al. 1997). Application of many factors such as climate change, plant tissues, soil type, and interaction with other microorganisms. These factors may affect the structure and species composition of the bacterial communities in plant tissues (Lacava et al. 2004; Mocali et al. 2003). The communities of endophytic antagonist bacteria inside the plant will be vary along with environment changes including the presence of phytopathogen.
Members of the genus Burkholderia sp. are known for their ability to suppress soil-borne fungal pathogens by the production of various antibiotic compounds such as pyrrolnitrin and phenazines (Kirner et al. 1998). Other different antibiotics such 2,4-diacetylphloroglucinol (2,4-DAPG) and pyoluteorin has also been found responsible for suppression of soil-borne fungal pathogens (Subagio & Foster 2003).
Antibiotic-related genes can be detected from BCAs by using Polymerase Chain Reaction (PCR) by using antibiotic specific primers, which encode phenazine-1-carboxylic acid, 2,4-DAPG, pyoluteorin, and pyrrolnitrin, from
2
BCAs have been applied in many ways and on many crops species, for instance, seed dipping application on rice seeds to control bacterial blight disease caused by Xanthomonas oryzae was able to reduce the disease incidence (Suryadi
et al. 2012). Besides microbial pathogen, BCA was also reported able to control plant parasitic nematodes. Munif et al. (2013) was reported seed dipping application on tomato seeds able to control Meloidogyne incognita penetration and enhanced the plant growth. Oil palm germinated seeds dipping application was also conducted by Dikin et al. (2003) to suppress Schizopyllum commune, causal agents of brown germ and seed rot in oil palm.
Scientific Problem
1. G. boninense is the most serious disease of field palms in Southeast Asia, particularly Malaysia and Indonesia. Ganoderma BSR is now recognized as a significant constraint to sustainable production in Asia, and development of techniques for disease management has been highlighted as a key research priority.
2. Chemical pesticides usage is consider to polution risk. We need a greener alternative to control plant pathogen.
3. G. boninense growth can be supress by using biocontrol agent such as bacteria.
4. Burkholderia exhibit a antibiotic compounds which responsible for
antifungal. Many research have reported about using microbes as a biocontrol agent, but none showed a significant result in oil palm plantation. 5. Different climate and different land may give a different effect for
biocontrol agent. By using endophyte bacteria may minimize the environmental factor.
Objective
The aim of this research in general is to gain endophyte biocontrol agent potential in surpressing G. boninense growth in oil palm. Specific purposes of this study are (1) to detect antifungi encoding gene from Burkholderia potential, thus to gain information which antifungal compounds that play role in inhibiting G.
boninense growth, (2) to determine the effectiveness of endophytic Burkholderia
in suppressing G. boninense infection in-vivo and in vitro(3) to gain community profile of endophyte bacteria in general and Burkholderia specifically among the treatments with G. boninense as the plant pathogen.
Research Benefit
3
Research Scope
This research include isolation and genetic identification of Burkholderia
sp. from roots and soil taken from oil palm plantation. In vivo application of
Burkholderia sp. and G. boninense to oil palm seedling by using dipping treatment. Plant growth measurement and infection scoring of oil palm after 3 months planting. Bacterial genome isolation from oil palm roots after treated with
Burkholderia sp. and G. boninense and bacterial community profile analysis by using DGGE. Specific antifungal-related gene amplification by using PCR and analyse the sequence by using gene bank (www.ncbi.nlm.nih.gov).
LITERATURE REVIEW
G. boninense in Oil Palm
The world‟s palm oil demand increased sharply in the past 5 years. In 2009, global consumption for palm oil was 42 million tons, and in 2011 was 49.05 million tonnes, higher than rapeseed and soybean oil (Oil World 2012). The greatest threat to sustainable oil palm production is Southeast Asia is from basal stem rot disease caused by the white rot fungus G. boninense (Flood et al. 2000).
Most severe losses from BSR occur in Indonesia and Malaysia with lower incidences being recorded in Africa, Papua New Guinea and Thailand (Idris et al.
2004). In North Sumatra, Indonesia, by the time of replanting (25 years) 40-50% of palms are lost with the majority of standing palms showing disease symptoms. The more serious palm losses due to G. boninense is occur in plantation where the oil palm stumps were left in the ground after replanting (up to 25% occurred within 7 years) (Subagio & Foster 2003).
Losses begin to have a financial effect once the disease affects more than 10% of the stand (Hasan & Turner 1998). On average there is a decline of the yield of the fresh fruit bunch (FFB) of 0.16t/ha for every palm lost, and when the stand had declined by 50% the average FFB yield reduction was 35% (Subagio & Foster 2003). Plant improvement is required to overcome this problem.
4
Basal Stem Rot Disease
Basal stem rot disease (BSR) caused by G. boninense is currently the major disease in oil palm plantations (Darmono 1998). Typically the fungus may attack an already weakened oil palm as G. boninense is seldom infects healthy trees seriously (Paterson 2007). Wong et al. (2012) mentioned that infection mainly occur in palms aging 30 years and above. The infections in younger palms of 10-15 years become more apparent, followed by spreading of the disease in oil palms at nursery stage.
G. boninenseis a white rot fungus. The term „„white rot‟‟ derives from the
fungus degrading specifically the lignin component of wood while leaving white cellulose exposed (Paterson 2007). Lignin biodegradation is probably a major part of the disease process. Lignin protects the more amenable cellulose and hemicelluloses from enzymatic attack by forming direct chemical bonds. White rot fungi such as G. boninense, are an organism that has the capability of degrading lignin into carbon dioxide and water, then celluloses is available as nutrients for the fungus.
The earliest external symptoms of BSR of oil palms occur in the foliage. In young palms, external symptoms of BSR comprise a yellowing or mottling of the lower fronds, followed by necrosis. Young unfolded leaves become chlorotic and may be reduced in length, sometimes with necrotic tips (Corley & Tinker 2003). As the disease progresses, palms may take on a pale appearance, with retarded growth and spear leaves remaining unopened. Similar symptoms are observed in mature palms, with multiple unopened spear leaves and a generally pale leaf canopy. Ultimately, affected palms may died, the necrosis beginning with the oldest fronds and extending to younger regions of the crown. Palms normally die within 6 to 12 months after the appearance of unexpanded spear leaves. The infection of G. boninense on plant can be scored by using disease severity index (DSI) showed in Table 1. (Abdullah et al. 2003).
Table 1 Score and disease scale of G. boninense infection in oil palm Disease
class Sign and symptom infection
0 mycelium on any part of plants
Appearance of white fungal mass on any part of plants, with or without chlorotic leaves
Appearance of basidioma on any part of plants with chlorotic leaves (1-3 leaves)
Formation of basidioma on any part of plants with chlorotic leaves (> 3 leaves)
Formation of well-developed basidioma and the plants dried Endophyte Microbes
5
almost every part of biotic and abiotic. Microbes which lived inside the plant tissue is called endophyte. The usage of this term is equally for variable strategies of symbiosis, ranging from facultatively saprobic to parasitic to exploitive to mutualistic. It is because if taken literally, it can include all pathogen at some stage of their development, since the plant host responds to at least some infection with mechanical defence reactions (Narisawa et al. 2004).
Petrini et al. (1991) add another characterisation of endophytic interactions as not "causing apparent harm", which presumably refers to an absence of macroscopically visible symptoms. Aware of the determinative discrepancies, the term of endophyte here to describe is those bacteria that can be detected at a particular moment within the tissues of apparently healthy plant hosts. Particular moment is added in term of endophyte because of the associated habitat is dynamic along with environment changes, including the presence of pathogen fungi.
As its ability to live inside the plant, endophytic bacteria can be utilized as sustainable biocontrol. Introducing endophytic potential bacteria into plant tissue has been widely applied by dipping the seeds or roots of plant in bacterial culture concentrate. This treatment will increased the number of endophytic bacteria inside the plant, but will not stay stable in a long time. Krechel et al. (2002) reported that after treatment, in the first 3 weeks after seedling the density of endophytic microbe will increased up to 105 cfu.g-1, and this number will stay constant through plant growth periode. Plants has its own mechanism to tolerate some number of endophyte living inside, and able to stabilize the number through competition.
The population of endophytic itself is not spread evenly in all parts of the plant. Hallman et al. (1997) reported that the population densities of indigenous endophytic bacteria in roots are found about 105 cfu.g-1 fresh root weight. This is higher than any other plant organ with average densities of 104 cfu.g-1 and 103 cfu.g-1 fresh weight in stem and leaves respectively.
Potential Endophyte Bacteria
Endophyte bacteria living inside the plant and bring an advantages to the plant host. It has a direct and indirect role in plant growth and plant defence system. Some bacteria exhibit a broad range of antifungal compound, and some regulate plant growth hormone. Schimidt et al. (2009) reported that antibiotic compounds produced by Burkholderia sp. are lipopeptides, cepaciamides A and B, cepacidines, siderophores, altericidin, pyrrolnitrin, glidobactins, phenazines and 2-hydroxymethyl-chroman-4-one. Antibiosis has been widely studied as one of the most important biocontrol mechanisms inhibiting plant pathogens.
6
regions for polymerase chain reaction (PCR) detection of antibiotic producing bacteria.
DGGE Analysis of Endophyte Community
Arau´jo et al. (2002) worked on diversity of endophytic bacterial population in healthy, resistant, and Citrus Variegated Chlorosis (CVC)-affected citrus plants. He was using cultivation and cultivation-independent techniques to assess the endophytic communities. His work showed that the diversity of endophytic bacterial population in each treatment were different. The result showed there were some specific bands that only appeared in asymptomatic plants. Also from the cultivation observed, some isolates were found significantly more frequent from asymptomatic plants than the other treatment. This came to a suggestion that this organism has a role in the resistancy of plants to CVC. His research proved that community of endophytic bacteria is influence by environment.
Bacterial community can be observed by using several methods, common method that is used are DGGE (Denaturing Gradient Gel Electrophoresis), T-RFLP (Terminal- Restriction Fragment Lenght Polymorphism), and SSCP (Single Strand Conformation Polymorphism). Smalla et al. (2007), compared DGGE, T-RFLP, and SSCP for assessing bacterial diversity in soil. The result showed although the fragments amplified comprised different variable regions and lengths, DGGE, T-RFLP and SSCP analyses led to similar findings. The clustering of fingerprints which correlated with soil physico-chemical properties is similar. Also the variability between the four replicates of the same soil is small. The results showed that the 3 method is stable and show similar result. DGGE is used in this research to assess the diversity of bacterial after introduced with endophytic bacteria biocontrol potential and planted in treated soil.
DGGE is a molecular fingerprinting method that separates polymerase chain reaction (PCR)-generated DNA products. However, since PCR products from a given reaction are of similar size (bp), conventional separation by agarose gel electrophoresis results only in a single DNA band that is non-descriptive. DGGE can overcome this limitation by separating PCR products based on sequence differences that results in differential denaturing characteristics of the DNA. During DGGE, PCR products encounter increasingly higher concentrations of chemical denaturant as they migrate through a polyacrylamide gel.
7
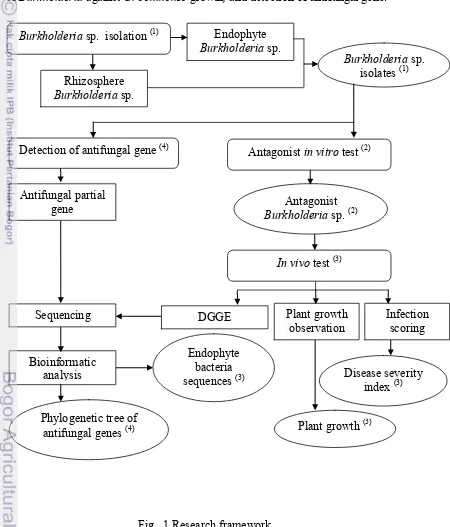
METHOD
Research framework
The experiment was divided into four steps as shown in Fig. 1. The research activity was consist of bacterial isolation, antagonist in vitro test and in vivo test of Burkholderia against G. boninense growth, and detection of antifungal gene.
Fig. 1 Research framework
Burkholderia sp. isolation (1)
Disease severity Detection of antifungal gene (4)
8
Research Time and Place
This research was start from June 2013 and finish on February 2014. Laboratory activity was took place in laboratory of Microbiology, Biology Departement, IPB, Bogor and laboratory of Microbiome Technology, PT. SMART Tbk, Sentul, Bogor. plate. The plate were incubated for 24-36 hours. Each of the grown colony were subculture and genome extracted for identification. Purified PCR product of 16s DNA were sent to 1st Base, Singapore.
Antagonist in vitro test
Antagonist test was observed to determine the percentage inhibition of radial growth (PIRG) of G. boninense (Bivi et al. 2010). Six bacterial isolates of
Burkholderia sp. were selected to evaluate their efficacy in enhancing growth and inhibit the infection of BSR in oil palm in pre nursery. There were five rhizosphere Burkholderia sp. (B313, B51a, B52c, B51b, B52a) and one endophyte
Burkholderia sp. (B212).
Burkholderia sp. was streaked into the PDA plate 2.5 cm from the edge of
the Petri dish. Agar disc cut diameter 5 mm of 5-day old G. boninense was placed 2.5 cm from the edge at the opposite side of the same Petri dish. For the control plate, only G. boninense was placed in a similar manner without bacteria on a fresh Petri dish. The plates were incubated at 28oC for five days.
Results shown by measured the radial growth of G. boninense. PIRG was measured using the equation below (Zaiton et al. 2006):
PIRG : percentage inhibition of radial growth; R1 : radial growth of G. boninense in the absence of bacteria (control); R2 : radial growth of G. boninense
in the presence of Burkholderia sp. The three highest PIRG isolates from in vitro
test were used for in vivo test.
In vivo test
Burkholderia sp. suspensions were prepared by inoculating 24 h-old
cultures into Nutrient Broth (NB) and incubated for 20 hr and adjusted to 108 cfu. mL-1. During the preparation of mixture, equal volume of the three highest PIRG
9
cm sterilized oil palm fronds. The fronds were sterlized in a heat resistant plastic each before inoculated with G. boninense. After sterlized, the fronds were inoculated with a 0.5 cm diameter disc of G. boninense mycellia on agar plate. The plastic was sealed and incubated in room temperature for 3 months before used.
The plant material was Tenera oil palm and provided by PT. SMART Tbk. The plant material was selected as the most G. boninense susceptible progeny. Two different progenies were used in this research. Briefly, oil palm germinated seeds were treated with bacterial suspension (108 cfu. mL-1), dipped for 20 min (seed bacterization) and air dried for 10 min before planted.
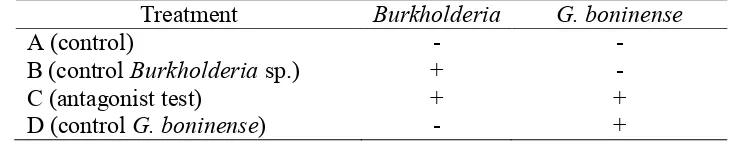
Oil palm germinated seeds were planted in polybags (15 cm x 20 cm) regarding to the Standard Operation Prosedure (SOP) in prenursery of oil palm plantation. There were four treatments in this research as shown in Table 2. Treatment A and C were not inoculated with G. boninense and treatments C and D were inoculated with G. boninense. Treatment B and C were using seedling treated with Burkholderia sp. Treatments with G. boninense, the seedlings were placed in contact with radicula. The pots were placed under shead, watered daily and no supplementary organic fertilizer was applied for 3 months (prenursery). A destructive observation was conducted after 3 months.
Table 2 Treatments of Burkholderia sp and G. boninense on oil palm seedling, Treatment Burkholderia G. boninense
A (control) - - signs and symptom on the treatment plants using disease severity index (DSI) as in Abdullah et al. (2003). DSI was observed from the external symtomp from foliar and the roots (destructive method).
The score can be calculated by the formula of Nur Ain Izzati and Abdullah (2008) as below in percentage :
where:
A : disease class (0, 1, 2, 3 or 4)
B : number of plants showing that disease class per treatment
10
Bacterial genome isolation
The roots (1g) which already surface sterilized, were placed in a mortar and grinded into fine powder by using liquid nitrogen. Bacterial genome were islated by using PowerPlant DNA isolation kit (MoBio). Then visualized the total DNA by electrophoresis on a 1% (wt/vol) agarose gel with 100 volt, 30 min. PCR-DGGE Analysis and Sequencing DGGE method. PCR products obtained from 16S rDNAs with the 357F (5´-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC CTA CGG GAG GCA GCAG-3´) and 518R (5´-ATTACCGCGGCTGCTGG-3´) primers developed by Muyzer et al. (1993). The DGGE-PCR was carried out with the temperature profile as follows-an initial denaturation step of 3 min at 95oC, followed by 35 cycles of 94oC for 1 min, 55oC annealing for 30 sec, 72oC pprimer extention for 1 min and a final step was carried out 72oC for 7 min. PCR product was analyzed by electrophoresis in a 1% (wt/vol) agarose gel with 0.5 x TAE buffer and stored at 20°C for DGGE analysis.
DGGE was performed on PCR products obtained as above using the D-Code universal mutation detection system as per the manufacturer's instructions (Bio-Rad, USA). PCR samples were loaded into 8% (wt/vol) polyacrylamide gels in 1 x TAE buffer (20 mM Tris-acetate, 0.5 mM EDTA, pH 7.4). The polyacrylamide gels were made with denaturing gradients ranging from 30 to 70% (where the 100% denaturant contained 7 M urea and 40% formamide). The gels were run for 6 h at 150 V and 60°C, after which the gels were soaked for 1 h in SYBR Green I nucleic acid stain (1:10,000 dilution; Molecular Probes, Leiden, The Netherlands) and immediately photographed under UV light.
Prominent bands are excised from the gels, reamplified, and subjected to DGGE as previously described. The new PCR products were purified with GenJET PCR purification kit (Thermo Scientific). Analyses of sequences are performed with the basic sequence alignment (BLAST) program run against the BLAST database (National Center for Biotechnology Information website [http://www.ncbi.nlm.nih.gov]).
Detection antifungal gene
The DNA isolation was carried out by using GeneJET Genome DNA Purification Kit from Thermo Scientific. Burkholderia sp. was prepared in 10 mL liquid mediumand the cells were collected after incubated for 18 hr in 37oC.
11
Table 3 Polymerase chain reaction primers and expected amplification products from genes encoding enzymes involved in the biosynthesis of several antibiotics.
Primer Antibiotic Sequence Sequence
size (bp)
12
RESULTS AND DISCUSSION
Results
Burkholderia sp. isolation
Bacterial isolation from root and soils were found five isolates of rhizosphere Burkholderia sp. and one isolate of endophyte Burkholderia sp. The isolates were rod shape and Gram negative cell on 1000x magnification under microscope. The colony was yellowish translucent on Nurtient agar (NA) media (Fig. 2).
Fig. 2 Burkholderia sp. growth on nutrient agar (NA) plate.
Antagonist in vitro test
All of the Burkholderia sp. isolates showed an inhibition activity against G. boninense growth in vitro. Burkholderia B212 showed the highest inhibition on
G. boninense growth in vitro (Fig. 3).
Fig. 3 In vitro test of Burkholderia sp. against G. boninense growth. R1 as the control and R2 is the radial growth of G. boninense on trial with
Burkholderia B212.
13
significantly different with B212, but still has an antagonist activity against G. boninense growth in vitro.
Table 4 Percentage of inhibition ratio from Burkholderia sp against G. boninense
growth in-vitro.
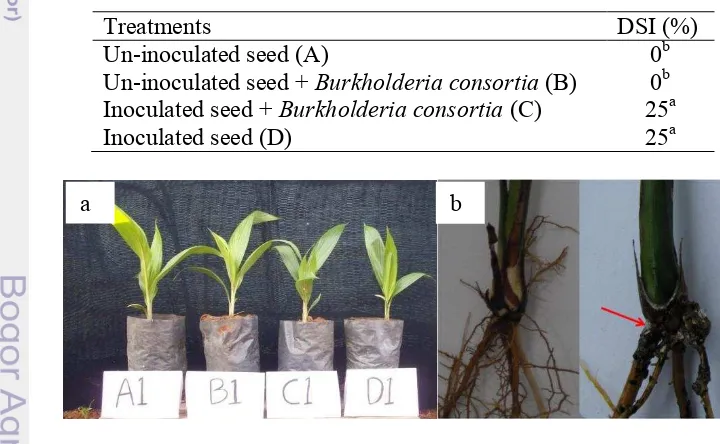
The necrosis and chlorosis foliar was not seen in all treatments, though the height of the plants between treatment was seem different (Fig. 4a). DSI after 3 months only showed in treatment C and D (25%) (Table 5). Destructive observation showed that all plants in treatment C and D were infected with G.
boninense. Treatment C and D, which were inoculated with G. boninense, showed
a brown-blackening roots especially on the parts which colonized with the G.
boninense (Fig. 4b). The roots in treatment A and C, without G. boninense were
cream-brown colored.
Table 5 Disease severity index (DSI) of treated plant with G. boninense.
Treatments DSI (%)
Un-inoculated seed (A) 0b
Un-inoculated seed + Burkholderia consortia (B) 0b Inoculated seed + Burkholderia consortia (C) 25a
Inoculated seed (D) 25a
Fig. 4a. Plants treatment left to right (A: control, B: control Burkholderia sp., C: antagonist test, D: control G. boninense). b. Healthy root with no appearance of fungal mycellia (left). Appearance of fungal mycellia (red arrow).
14
Progeny give a significant different mostly to the shoot lenght and dry weight (Table 6). Total number of roots between progeny also showed a significant difference. However, there is no interaction between progenies and treatments though in some parameter that were observed was showed a significant difference.
Table 6 The effect of different progeny on root and leaf total number, lenght, and dry weight after treatment with G. boninense and Burkholderia sp.
Progeny Lenght (cm) Dry weight (g) Total number of
Burkholderia sp. consortia application on inoculated seeds showed
significantly lowest in shoot growth compare to control and other treatments. In all parameters that were measured, the treatment inhibited the plant growth, and significantly decrease shoot lenght, shoot dry weight, total number of shoot and root (Table 7). However, Burkholderia sp. consortia application on un-inoculated seeds showed the highest in shoot and root growth compare other treatments. The shoot lenght and dry weight was slightly different compare to control. The root lenght did not showed a significant different among all the treatment.
Table 7 The effect of dipping treatment of antagonistic bacteria on seedling of oil palm inoculated with G. boninense in pre nursery at 3 months after planting Treatment Lenght (cm) Dry weight (g) Total number of
Shoot Root Shoot Root shoot root
Primer for PCR-DGGE generates a 161bp lenght of 16s DNA partial (Fig. 5), which were design to amplify v3-variable region of 16s DNA. Genome of pure
15
Fig. 5 PCR amplification of 16S rRNA gene from oil palm roots after 3months.
Separation of 16s DNA by using DGGE showed that there were no significant variation in pattern between treatments. Treatment a, c, and d showed a similar pattern on DGGE gel, it showed four different bands, while treatment b only showed a single band. Control Burkholderia (Burk) showed a single band on DGGE gel (Fig. 6). The bands were sequenced and showed that band 1 (RFN1), which is appear on sample a, b, and d, was similar with Elaeis oleifera with 90% maximum identity.
Fig. 6 DGGE band profile of endophyte bacteria treatment with Burkholderia and
G. boninense (left), lane a: control, lane b: control Burkholderia sp., lane c: antagonist test, lane d: control G. boninense, lane Burk: isolate Burkholderia
B212. Cluster analysis of endophyte bacterial in oil palm root treated with
Burkholderia sp and G. boninense with 1D Pro Phoretrix software (right). Lane 1 = lane a, lane 2 = lane b, lane 3 = lane c, lane d = lane 4, lane 5 =
Burk.
Band 2 (RFN2) was appear on all sample and it was sequenced as uncultured bacterium with 99% maximum identity. Band 3 (RFN3) was a very thin band under the RFN2 band, it was not found with any similar sequence in NCBI, but it has 61% similarity with band RFN1 in phylogenetic tree (Fig. 7). Bands 4, 5, and 6 (RFN4, RFN5, and RFN6) was sequenced as uncultured bacteria DGGE with 97% maximum identity.
16
Fig. 7 Phylogenetic tree of excised bands from DGGE.
Detection of antifungal gene and sequence analysis
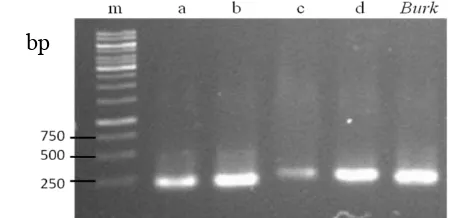
Primers PRND1 and PRND2 amplified the predicted 790-bp fragment from DNA of B212, B313, B51a, and B52c. Primers PLTC1 and PLTC2 amplified the predicted 438-bp from DNA of Burkholderia B313, B51a, and B52c (Fig. 8). Primers for phenazine and DAPG did not yield a PCR product from all isolates. This may be conclude that these isolates do not contain phenazine and DAPG biosynthesis gene.
Fig. 8 PCR product of pyrrolnitrin and pyoluteorin gene amplification. M : marker, 3. prn primer, 4. plt primer, a. B212, b. B313 , c. B51a , d. B52c , e. B51b, f. B52a. Red arrows showed the expected band size.
The PCR product were BLAST and showed a 99% identity with B. cepacia
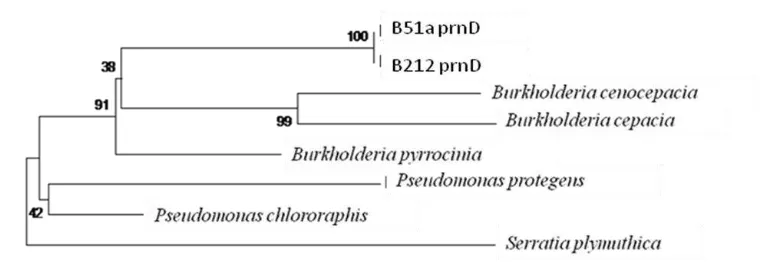
partial prnD gene, strain ESR63 (Fig. 9). It is confirmed B212 has a potency to produce pyrolnitrin and assumed pyrolnitrin is the responsible compound in inhibiting G. boninense growth in vitro. The sequence of PRND partial gene is attached (attachment 3) by the end of this thesis.
17
Fig. 9 Phylogenetic tree of PrnD gene from B212 and B51a.
Discussion
Bacteria have various mechanism of antagonistic such as synthesizing antibiotic compounds, production of hydrolytic enzymes, siderophore production, competition for substrates and also induction of systemic resistance in the host plant will increase the plant resistance to a broad spectrum of pathogens (Kloepper & Ryu 2006). Many soil-borne plant diseases caused by fungi and oomycetes can be controlled by strains of the genus Burkholderia sp. (Kirner et al. 1998).
In this research endophyte Burkholderia sp. B212 showed the highest antagonist activity against G. boninense growth (Table 2). Several strains of
Pseudomonas and Burkholderia species can produced a broad-spectrum of
antibiotics which play an important role in the suppression of multiple plant pathogenic fungi (de Souza & Raaijmakers 2003).
In vivo test showed a different result from the in vitro test. In comparison to Izzati and Abdullah (2008) and Zaiton et al. (2008), the external symptom of G. boninense infection in oil palm appeared after 4 months after planting even though the disease has been invested from seedling or after 3 months planting. However, external symptom may also occur after 2 months planting with the disease.
18
fronds are more easy to get since it is abundance in oil palm plantation. Different climate may also effect lignin degradation, which lignin degradation is less efficient at 37oC compare to 25o C (Paterson et al. 2008).
Application method of Burkholderia sp. may also effect the result of infection. Burkholderia sp. has been reported that its antifungal producing is related to quorum-sensing, which is mean that the antifungal activity depends on
Burkholderia sp. biomassa (Chapalain et al. 2013). G. boninense infection were found in all plants which were treated with the disease including the Burkholderia
sp. consortia treatment. Burkholderia sp. consortia were expected to inhibit the disease infection, but the result showed on the contrary. It is very different to other report which is using soil drenched application and the result showed that application with Burkholderia and Pseudomonas able to reduce G. boninense
incidence (Zaiton et al. 2008). Different method of application and number of
Burkholderia sp. applied may come to a different results.
Burkholderia sp. consortia application on inoculated seeds was expected to
have the highest plant growth compare to plants without Burkholderia sp. and control. However, the treatment showed the lowest plant growth compare to all other treatments. Burkholderia sp. as endophyte has the ability to penetrate the plant by using cellulase enzyme (Reinhold-Hurek et al. 2006). It come to assumption that the Burkholderia sp. has a potency to open a way for G. boninense to infect the plants. Biomass of Burkholderia sp. held an important key in suppress G. boninense infection, since the antifungal compound is regulated by quorum sensing. This fenomena could explain the infected plants without
Burkholderia sp. consortia application is higher than the infected plants with
Burkholderia sp. consortia.
If compared with the shoot and root ratio (S/R) among the treatment, infected plants with Burkholderia sp. consortia shown the lowest number. Which means that in this treatment, the root has the higher biomass compare to the shoot.
Burkholderia sp. consortia application gave a more affect to the roots than the shoots on infected plants. Burkholderia sp. also could acted as PGPR which can lead an indirect biocontrol agent, since it could enhanced plant growth.
Burkholderia has reported as a plant growth promoting rhizobacteria (PGPR) (Compant et al. 2007).
Burkholderia sp. consortia application on inoculated seed was significantly higher on root dry weight compare to other treatment. G. boninense as pathogen could induced plant defense by forming lignin as its first defence system. Lignification or cell thickness is the form of plant defense against pathogen (Xu et al. 2011) and could increase root biomass .
Total number of Burkholderia applied also play an important role in proper activity of this biocontrol agent. Schmidt et al. (2009) has reported that some of antifungal such as pyrrolnitrin is regulated by quorum sensing. Quorum sensing is one mechanism to regulate the production of antimicrobial compounds by population-density-dependent (Liu et al. 2007). Different result between in vitro
and in vivo test may be caused by many factors such as plant ages by the time artificially inoculated, concentration of microbes applied, and application technique.
19
community profile was similar between all treatment. Compare to Situmorang et al. (2014), endophyte bacteria on oil palm was very vary. The difference was found on the sample that were use for DGGE. Situmorang et al. (2014) were use root sample from more than 10 years oil palm trees. The plant age is affect the endophyte community profile.
Many research showed that Burkholderia sp. could decrease G. boninense
infection on oil palm, but yet a correct application technique of this bacteria also important to get the best result. Antifungal compound is a secondary metabolite which is produce by bacteria in their stationary phase. Further research need to be conducted for the proper technique application of this Burkholderia sp. for its optimum action.
Pyrrolnitrin encoded gene was amplified and showed that Burkholderia
B212 has a potential in producing antifungal agent such as pyrrolnitrin. Pyrrolnitrin has been implicated as an important mechanism of biological control of fungal plant pathogens by several Pseudomonas strains (Hasan & Turner 1998). Pyrollnitrin is a chlorinated phenylpyrrole antibiotic that was first isolated from
Burkholderia pyrrocinia (Kloepper & Ryu 2006) and later from other
microorganisms, including Pseudomonas fluorescens, P. chlororaphis, P. aureofaciens, B. cepacia, Enterobacter agglomerans, Myxococcus fulvus, and Serratia species (Hammer et al. 1999).
Pyrrolnitrin is synthesize by four protein encoded by 4 gene, prnA, prnB, prnC, and prnD. PrnD gene was the final protein to form an active pyrrolnitrin compound. PrnD catalyzes the oxidation of the amino group of aminopyrrolnitrin to a nitro group to form pyrrolnitrin (Kirner et al. 1998). In some strains of
2) The highest antagonist activity was shown by isolate B212, with inhibition ratio 34.38%. In vivo test of Burkholderia in fungal inoculated plant showed a total absence in foliar symptom and 25% of DSI. In treatment
Burkholderia control (Burkholderia applied without G. boninense) could enhance plant growth and biomass. However, the treatment of Burkholderia
on fungal inoculated plant decreased the plant growth.
20
Sugestion
Another application method of endophyte Burkholderia should be conduct to determine the best method to control G. boninense in oil palm. The observation should be done longer, especially for profilling endophyte bacterial community. The antifungal compound of Burkholderia sp. should be extracted to be charactherized and for further research.
REFERENCES
Abdullah F, Ilias GNM, Nelson M, Nur Ain Izzati MZ, Umi Kalsom Y. 2003. Disease assessment and the efficacy of Trichoderma as a biocontrol agent of basal stem rot of oil palms. Res Bulletin Sci Putra 11: 31–33.
Arau´jo WL, Marcon J, Maccheroni JrW, Elsas JD, Vuurde JWL, Azevedo JL. 2002. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol. 68: 4906-4914.
Bivi MR, Farhana MSN, Khairulmazmi A, Idris A. 2010. Control of Ganoderma boninense: a causal agent of basal stem rot disease in oil palm with endophyte bacteria in vitro. Int J Agric Biol. 12:833–839.
Chapalain A, Vial L, Laprade N, Dekimpe V, Perreault J, Deziel E. 2013. Identification of quorum sensing-controlled genes in Burkholderia ambifaria. Microbiol open. 2: 226-242.
Compant S, Nowak J, Coenye T, Clement C, Barka EA. 2007. Diversity and occurence of Burkholderia spp in the natural environment. FEMS Microbiol Rev 1: 607-626.
Cooper RM, Flood J, Rees RW. 2011. Ganoderma boninense in oil palm plantations: current thinking on epidemiology, resistance, and pathology.
The Planter 87(1024):515-526
Corley RHV, Tinker PB. 2003. The Oil Palm, Fourth eds. Blackwell Publishing, p. 407-408.
Darmono TW. 1998. Ganoderma in oil palm in Indonesia: current status and prospective use of antibodies for the detection of infection. In: Harman GE, Kubicek CP, eds. Trichoderma and Gliocladium Volume 1: Enzymes, biological control and commercial applications. UK: Taylor & Francis Ltd, p. 393.
de Souza JT, Raaijmakers JM. 2003. Polymorphisms within the prnD and pltC genes from pyrrolnitrin and pyoluteorin-producing Pseudomonas and
Burkholderia spp. FEMS Micobiol Ecol 43: 21-34.
Dikin A, Sijam K, Ahmad ZAM., Seman, I.A. 2003. Biological control of seedborne pathogen of oil palm, Schizopyllum commune Fr. With antagonistic bacteria. Int J Agri Biol 5: 507-512.
Flood J, Bridge PD, Holderness M. 2000. Ganoderma Diseases of Perennial
Crops. CABI Publishing, Wallingford. (UK) p. 275
21
Haas D, Défago G. 2005. Biological control of soil-borne pathogens by fluorescent Pseudomonads. Nat Rev Microbiol 3: 307-319.
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. 1997. Bacterial endophytes in agricultural crops. Can J Microbiol. 43:895-914.
Hammer PE, Burd W, Hill DS, Ligon JM,Van Pée KH. 1999. Conservation of the pyrrolnitrin gene cluster among six pyrrolnitrin-producing strains. FEMS Microbiol Lett 180: 39-44.
Hasan Y, Turner PD. 1998. The comparative importance of different oil palm tissues as infection sources for basal stem rot in replantings. The Planter
74: 119-35.
Homma Y, Sato Z, Hirayama F, Konno K, Shirahama H, and Suzui T. 1989. Production of antibiotics by Pseudomonas cepacia as an agent for biological control of soilborne plant pathogens. Soil Biol Biochem.
21:723–728.
Kirner S, Hammer PE, Hill DS, Altmann A, Fischer I, Weislo LJ, Lanahan M, van Pée KH, Ligon JM. 1998. Functions encoded by pyrrolnitrin biosynthetic genes from Pseudomonas fluorescens. J Bacteriol 180(7):1939-1943. Krechel A, Faupel A, Hallman J, Ulrich A, Berg G. 2002. Potato associated bateria
and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid and White) chitwood. Can J Microbiol. 48:772-786.
Kloepper JW, Ryu CM. 2006. Bacterial endophytes as elicitors of induced systemic resistance. In : Schulz B, Boyle C, Sieber TN. (Eds), Microbial Root Endophytes, Soil Biology, Vol.9. Springer-Verlag Berlin Heidelberg, p. 33-52.
Lacava PT, Arau´ jo WL, Marcon J, Maccheroni Jr W, Azevedo JL. 2004. Interaction between endophytic bacteria from citrus plants and the phytopathogenic bacteria Xylella fastidiosa, causal agent of citrus-variegated chlorosis. Lett Appl Microbiol. 39: 55–59.
Liu X, Bimerew M, Ma Y, Müller H, Ovadis M, Eberl L, Berg G, Chernin L. 2007. Quorum-sensing signaling is required for production of the antibiotic pyrrolnitrin in a rhizospheric biocontrol strain of Serratia phymuthica. FEMS Microbiol Lett. 270: 299–305.
Mocali S, Bertelli E, Celli F D, Mengoni A, Sfalanga A, Vilani F, Caciotti A, Tegli S, Surico G and Fani R. 2003. Fluctuation of bacteria isolated from elm tissues during different seasons and from different plant organs. Res in Microbiol.154:105–114.
Munif A, Hallman J, Sikora RA. 2013. The influence of endophytic bacteria on Meloidogyne incognita infection and tomato plant growth. J ISSAAS
19(2):68-74
Muyzer G, De Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59: 695-700
Narisawa K, Usuki F, Hashiba T. 2004. Control of Verticullum yellows in chinese cabbage by the dark septate endophyte fungus LtVB3. Phytopathol.
94:412-418.
22
oil palm seedlings treated with Trichoderma harzianum. Plant Protec Sci
44: 101–107.
Oil World, 2012. Oil World Statistic Update. ISTA Mielke GmbH. Hamburg.pp 1.
Paterson RRM. 2007. Ganoderma disease of oil palm-A white rot perspective necessary for integrated control. Crop Protect. 26: 1369-1376
Paterson RRM, Meon S, Zainal AMA, Lima N. 2008. Prospects for onhibition of lignin degrading enzymes to control Ganoderma white rot of oil palm.
Curr Enzym Inhib. 4: 172-179
Petrini O. 1991. Fungal endophyte of tree leaves. In: Andrews J, Hirano S (eds)
Microbial ecology leaves. Springer, New York Berlin Heidelberg.
p179-197.
Reinhold-Hurek B, Maes T, Gemmer S, Van Montagu M, Hurek T. 2006. An endoglucanase is involved in infection of rice roots by the not-cellulose-metabolizing endophyte Azoarcus sp. strain BH72. Mol. Plant-Microbe Interact. 19: 181-188.
Schmidt S, Blom JF, Pernthaler J, Berg G, Baldwin A, Mahenthiralingam E, Eberl L. 2009. Production of the antifungal compound pyrrolnitrin is quorum sensing-regulated in members of the Burkholderia cepacia complex.
Environ Microbiol 11(6):1422-37.
Smalla K, Oros-Sichler M, Milling A, Heuer H, Baumgarte S, Becker R, Neuber G, Kropf S, Ulrich A, and Tebbe CC. 2007. Bacterial diversity of soil assessed by DGGE, T-RLFP and SSCP fingerprints of PCR-amplified 16S rRNA gene fragments: Do the different methods provide similar results? J Microbiol Methods 69:470-479.
Stads GJ, Haryono Nurjayanti S. 2007. Agricultural R&D in Indonesia : Policy,
investments, and institutional profile. Agricultural Science and
Technology Indicators. International Food Policy Research Institute and Indonesian Agency for Agricultural Research and Development.
Subagio A, Foster HL, 2003. Implications of Ganoderma disease on loss in stand and yield production of oil palm in North Sumatra. Proceedings of the MAPPS Conference (Aug 2003). Kuala Lumpur.
Suryadi Y, Susilowati DN, Ruskandar A. 2012. Seed-dipping application of local endophytic bacterial consortium against bacterial leaf blight of rice. J Agrotrop 17(1): 7-13
Wong LC, Bong C-FJ, Idris AS. 2012. Ganoderma species associated with basal stem rot disease of oil palm. Amer J of ApplSci. 9(6): 879-885.
Xu L, Zhu L, Tu L, Liu L, Yuan D, Jin L, Long L, Zhang X. 2011. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus
Verticilium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J Experiment Bot P:1-15
Zaiton S, Sariah M, Zainal Abidin MA. 2006. Isolation and Characterization of Microbial Endophytes from Oil Palm Roots: Implication as Biocontrol Agents against Ganoderma. The Planter, 82: 587–97
Zaiton S, Meon S, Ahmad ZAM. 2008. Effect of endophytic bacteria on growth and suppression of Ganoderma infection in oil palm. Int J Agric Biol
23
24
ATTACHMENTS
Attachment 1 List of sequencing results from Burkholderia sp. isolate. a. Isolate B212
>1st_BASE_1625711_B7_1510_R.ab1
25
b. Isolate B52a
> 1st_BASE_1625713_B23_1510_R.ab1
26
c. Isolate B52c
>1st_BASE_1625715_B28_1510_R.ab1
27
Attachment 2 List of sequencing results from DGGE band slicing. RFN 1
>1st_BASE_1613054_RFN1_357_F2
TCCTGANCNGNCTAGATAGAGACTTAAATAACGATACCGAGCTCCTCGAGTCTGGTA ATTTCAATGCTCACAATCTAAATCCCTTAACGAGGATCCATTGGACGGCAAGTCTGTC GCCGCANCCGCGGTAATANTA
RFN2
>1st_BASE_1613055_RFN2_357_F2
GGNANNTCGCATGGGCGAAGCCTGACGGAGCAATGCCGCGTGGAGGTAGAAGGCCC ACGGGTCGTGAACTTCTTTTCTCGGAGAAGAAGCAATGACGGTATCTGAGGAATAAG CATCGGCTAACTCTGTGCCAGCAGCCGCGGTAATA
RFN3
>1st_BASE_1613055_RFN3_357_F2
GAAGCCTGATCGAGCAATGCCGCGTGAAGTAGAAGGCCCACGGCTCGTGAACTTCTT TTCTCGCAAAAGAACCATTGACAGTATCTGAGGAATAAACGTCCCCTAACTCTCCGCC CCCAGCCGCGCTAATATA
RFN4
>1st_BASE_1613057_RFN4_357_F2.ab1
CCTGGGAACTGGAATGGGCGAAGCCCGATCCGCAATATCGCGTGAGTGAAGAAGGGC AATGCCGNCTTGTAAAGCTCNTT
TCGTCGAGTGCGCGATCATGACAGGACTCGAGGAAGAAGCCCCGGCTAACTCCGTGC CAGCAGCCGCGGTAATAA
RFN5
>1st_BASE_1613058_RFN5_357_F2
CCTGGGGAATTGGAATGGGCGAAGCCCGATCCAGCAATATCGCGTGAGTGAAGAAGG GCAATGCCGNCTTGTAAAGCNTCTTTCGTCGAGTGCGCGATCATGACAGGACTCGAGG AAGAAGCCCCGGCTAACTCCGTGCCAGCAGCCGCGGTAAT
RFN6
>1st_BASE_1613059_RFN6_357_F2
28
Attachment 3 Sequencing result of PRND gene. >1st_BASE_1302993_B3_PRND1.ab1
29
31
BIOGRAPHY
The author was born in Oklahoma U.S.A on the 17th October 1986 from father Dr. Lalang Buana M.Sc and mother Dr. Tri Haryati M.Si. The author is first daughter from 3 daughters.
The author took her bachelor degree in Bandung Institute of Technology and graduated in major Microbiology, School of Life Science and Technology, in 2009. The author works as a junior scientist in PT. SMART Tbk. in Microbiome Technology since October 2009 until present.
In year 2010, the author attended an international seminar of Indonesian Society for Microbiology in Bogor where she and her team presented a poster entitled “Isolation and characterization endophytic chitinase-producing fungi as biological control of Oil Palm Basal Stem Rot Caused by Ganoderma boninense” and also presented a poster entitled “Chitinolytic activity of endophytic bacteria isolated from Oil Palm roots and for Ganoderma boninense biocontrol” in International Oil Palm Conference in the same year.
In year 2011, the author attended the 7th Asian Crop Science Conference in Bogor where she presented a paper entitled “In vitro test of rhizosphere chitinolytic bacteria as a biocontrol for Ganoderma boninense”. The author also attended The 3rd International Conference on Oil Palm and the Environment held in Nusa Dua Bali and presented a poster entitled “Biodiversity of plant growth and health supporting microbes from PT. SMART Tbk plantation for sustainable oil palm production” in year 2012.
In year 2012, the author got the opportunity to further her study in Graduate School, Bogor Agricultural University in Microbiology, with funding from PT. SMART Tbk. During her time in the Graduate school, in year 2013, the author got the opportunity to join winter course program where she presented a paper in The 9th International Student Conference at Ibaraki University in Japan. The program held by Graduate School of IPB and Ibaraki University in Japan with funding from Ibaraki Univerisity, Japan.