A key role for experimental task performance: Effects of math talent, gender
and performance on the neural correlates of mental rotation
Christian Hoppe
a,⇑, Klaus Fliessbach
a,b, Sven Stausberg
a, Jelena Stojanovic
a, Peter Trautner
b,
Christian E. Elger
a,b, Bernd Weber
a,baDepartment of Epileptology, University of Bonn Medical Centre, Germany bDepartment of NeuroCognition, Life & Brain Centre, Germany
a r t i c l e
i n f o
Article history:
Accepted 18 October 2011 Available online 15 November 2011
Keywords:
Mental rotation Mathematical giftedness Gender effects
Neural correlates of cognitive performance Inferior parietal lobule
Functional magnetic resonance imaging
a b s t r a c t
The neurophysiological mechanisms underlying superior cognitive performance are a research area of high interest. The majority of studies on the brain–performance relationship assessed the effects of capa-bility-related group factors (e.g. talent, gender) on task-related brain activations while only few studies examined the effect of the inherent experimental task performance factor. In this functional MRI study, we combined both approaches and simultaneously assessed the effects of three relatively independent factors on the neurofunctional correlates of mental rotation in same-aged adolescents: math talent (gifted/controls: 17/17), gender (male/female: 16/18) and experimental task performance (median split on accuracy; high/low: 17/17). Better experimental task performance of mathematically gifted vs. control subjects and male vs. female subjects validated the selected paradigm. Activation of the inferior parietal lobule (IPL) was identified as a common effect of mathematical giftedness, gender and experimental task performance. However, multiple linear regression analyses (stepwise) indicated experimental task per-formance as the only predictor of parietal activations. In conclusion, increased activation of the IPL rep-resents a positive neural correlate of mental rotation performance, irrespective of but consistent with the obtained neurocognitive and behavioral effects of math talent and gender. As experimental performance may strongly affect task-related activations this factor needs to be considered in capability-related group comparison studies on the brain–performance relationship.
Ó2011 Elsevier Inc. All rights reserved.
1. Introduction
Interindividual variance of performance in a given task (e.g. accuracy, speed) is a ubiquitous psychological phenomenon. Any functional or structural brain property which co-varies with task performance can be addressed as aneural correlate of performance
(NCP) of the respective task. Searching NCPs is currently a highly active field in neurocognitive research (Deary, Penke, & Johnson, 2010; Haier, 2009; Neubauer & Fink, 2009; Rypma & Prabhakaran, 2009).
Neuroimaging research on NCPs has largely focused on the ef-fects of capability-related group factors (e.g. intelligence, talent, or expertise) on task-related brain activations (Grabner, Neubauer, & Stern, 2006; Lee et al., 2006; Singh & O’Boyle, 2004). Capability effects have been reported since the very beginning of functional neuroimaging (Charlot, Tzourio, Zilbovicius, Mazoyer, & Denis,
1992). Since stimulation tasks which address the specific knowl-edge or skills of high-capability subjects are beyond reach for stan-dard subjects, the applied tasks usually refer to elementary cognitive abilities that presumably contribute to the respective capability of interest. However, it is debatable whether a capabil-ity-related group effect on experimental task performance (i.e. behavioral performance during scanning) validates the supposed capability-task relationship or rather confounds the group factor (Bell, Willson, Wilman, Dave, & Silverstone, 2006; Butler et al., 2006; Jordan, Wustenberg, Heinze, Peters, & Jancke, 2002; Larson, Haier, LaCasse, & Hazen, 1995; O’Boyle et al., 2005; Thomsen et al., 2000; Unterrainer, Wranek, Staffen, Gruber, & Ladurner, 2000; Weiss et al., 2003a,b). In case of equal experimental task per-formance, neural correlates of the capability-related group factor do not represent an NCP of the applied task (according to the above definition) but rather indicate unknown group-specific neurocog-nitive factors which are irrelevant with regard to experimental task performance (e.g. stress response).
Alternatively, studies on NCPs may focus more directly on the effects of behavioral performance in an experimental task on the brain activations which were elicited by this very task. Task
0278-2626/$ - see front matterÓ2011 Elsevier Inc. All rights reserved.
doi:10.1016/j.bandc.2011.10.008
⇑Corresponding author. Address: Department of Epileptology, University of Bonn Medical Centre, Sigmund-Freud-Straße 25, 53105 Bonn, Germany. Fax: +49 228 287 90 16172.
E-mail address:christian.hoppe@ukb.uni-bonn.de(C. Hoppe).
Contents lists available atSciVerse ScienceDirect
Brain and Cognition
performance effects on functional brain activation (and also con-nectivity) were repeatedly reported and actually challenge any too simplistic approach to functional brain mapping (Rypma et al., 2006; Tagaris et al., 1996b, 1997; Unterrainer et al., 2000, 2005). Evidently, this approach makes full use of the available behavioral and neurophysiological data obtained by functional neuroimaging. In addition, NCPs of a given task can be assessed in non-indicated standard subjects, for example by contrasting ret-rospectively identified highvs.low experimental task performers
(Rypma et al., 2006).
In the present fMRI study, we combined these two research strategies to allow their evaluation and comparison. Referring to several previous studies from other groups (O’Boyle et al., 2005; Unterrainer et al., 2000, 2004, 2005), we simultaneously examined the effects of math talent, gender and experimental task perfor-mance on brain activations during mental rotation. Mental rotation is one of the best studied paradigms in both experimental psychol-ogy and cognitive neuroscience since its introduction byShepard and Metzler (1971). On a behavioral level, both gender (Collins & Kimura, 1997; Kimura, 1996; Linn & Petersen, 1985; Lippa, Collaer, & Peters, 2010; Lubinski & Humphreys, 1990; Masters & Sanders, 1986, 1993; Moore & Johnson, 2008; Peters, 2008; Quinn & Liben, 2008; Voyer & Hou, 2006; Voyer, Voyer, & Bryden, 1995) and math talent (Casey, Nuttall, & Benbow, 1995; Hyde, 2005; O’Boyle, Ben-bow, & Alexander, 1995; Spelke, 2005) have shown reliable effects on mental rotation performance. Mental rotation reliably activates the posterior parietal cortices (PPC) which also play a key role for working memory and general intellectual functioning (Champod & Petrides, 2007, 2010; Jung & Haier, 2007; Zacks, 2008).
Unfolding the idea of specific neural mechanisms underlying better cognitive performance of a given task, we tested the follow-ing hypotheses:
(I) Mathematically gifted vs. control subjects and male vs. female subjects show better mental rotation performance.
(II)Effect of the experimental task performance factor: Activation
of the PPC is obtained as an NCP, i.e. a positive neural corre-late of mental rotation performance.
(III)Effects of capability-related factors: Math talent and gender yield activations of the PPC similar to the effects of experi-mental task performance as both are associated with better experimental task performance.
(IV) As the elicited neurocognitive activations are more inher-ently related to the experimental task, most of the variance of PPC activations can be explained by the experimental task performance factor.
2. Materials and methods
This study was approved by the Ethical Review Board of the Medical Faculty at the University of Bonn (No. 039/06). The study was carried out in accordance withThe Code of Ethics of the World
Medical Association (Declaration of Helsinki) for experiments
involving humans.
2.1. Subjects
The study included 17 adolescent mathematically gifted sub-jects (MATH) and 20 same-aged control subsub-jects (CON) between the ages of 15 and 18 without mathematical talent. Mathematical talent was assigned if the student was matriculated for Mathemat-ics at the University of Bonn while attending high school (which relies on the recommendation of their schools) or if a subject re-cently participated in the ‘Mathematical Olympiad’ at a federal state level (state of North-Rhine Westphalia; total in 2007:
N= 350 out of 16,000 participants on the community level). During
subject recruitment, we additionally aimed at an equal distribution of male and female subjects in both samples to implement gender as an independent second capability-related group factor. Due to technical artifacts in the MRI data, three control subjects had to be excluded from the final analysis.Table 1lists the characteristics of the included subjects. All subjects had normal or corrected-to-normal vision. Subjects were reimbursed for participation (10€/h)
and travel costs. All subjects and their parents gave written in-formed consent according to the rules of good scientific practice.
2.2. Task
The original Shepard–Metzler (SM) paradigm shows a pair of drawings of quasi-3D-figures, each of which is constructed out of 10 cubes rendered in two dimensions (‘‘3D figures’’) and requires a matching decision (identical vs. mirrored). In contrast, the Van-denberg–Kuse (VK) paradigm (Vandenberg & Kuse, 1978) which was applied in the majority of neuroimaging studies (Zacks, 2008) simultaneously shows one Shepard–Metzler figure as the target and four additional figures as probes and requires the sub-jects to select the one probe which matches the target though being spatially rotated (i.e. 4-alternatives forced choice;Peters & Battista, 2008; Peters et al., 1995). In this paradigm, identical stim-uli (i.e. 0°angular disparity condition), scrambled dot patterns de-rived from the figures, or black and white bars serve as the control stimuli.
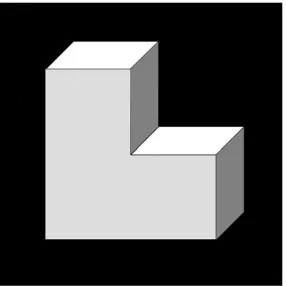
We propose a modified preparation rotation paradigm ( Jansen-Osmann & Heil, 2007): (i) To avoid the reportedly high error rates (>40%) of established paradigms (O’Boyle et al., 2005; Weiss et al., 2003a,b); (ii) to separate mental rotation proper from both the encoding of the stimulus and the matching test; and (iii) to im-prove experimental control over the task difficulty in terms of both cognitive load and speed demands for future studies. The modified paradigm is shown in Fig. 2. A two-dimensional rendering of a three-dimensional stair-like figure composed of three cubes (i.e. a fragment of the original Shepard–Metzler items;Fig. 1) was used as the stimulus. Rotations of this object had to be performed at 90° (instead of 15°) angles within one of the three spatial planes (hor-izontal, sagittal, frontal) resulting in 12 possible positions of the object. At the beginning of each trial, the stimulus was presented for 2 s in a randomly selected position. Subjects were then prompted to perform four continuous mental rotations of the ob-ject starting from the initial position as indicated by four serially presented arrows (duration: 13 s, i.e. 3.25 s per rotation; each ar-row presented for 0.813 s). Pilot studies in adolescent control sub-jects (N= 30) confirmed the appropriateness of these speed demands. In the task condition, the rotation plane was changed either for one time or for three times: InFig. 2, the task condition shows three rotation plane changes (upward/sagittal, right/hori-zontal, clockwise/frontal, downward/sagittal). In the control condi-tion (active low level task), the arrows indicated back-and-forth left and right rotating of the object within the horizontal plane (Fig. 2). The subjects were instructed that this condition only re-quired the maintenance of the figure in its initial position. Each trial was completed by a matching decision task presenting the ob-ject in one of the twelve possible positions as a probe. Subob-jects pressed a button to indicate if the probe matched the figure’s posi-tion after performing the instructed rotaposi-tions (response latency: 3 s). A total of 30 control and 30 task trials were presented alter-nately allowing mental rotation-related brain activations to return to the baseline. Matching decisions were recorded as correct or false responses. The individual mental rotation accuracy score,
MRX, was defined byMRX= (1 (number of errors during task
mental rotation proper performance controlled for maintenance performance.
2.3. Adjunctive behavioral measures
Nonverbal intelligence was estimated by theCFT 20-R, part 1 (Weiß, 2006), a German measure which is well-established for individual diagnosis and research on intellectual giftedness. The test comprises four subtests on visuospatial logical reasoning sim-ilar to Raven’s progressive matrices (test duration: 14 min.); a self-developed computerized protocol version showing copies of the original item sheets were used. Handedness was assessed by the
Edinburgh Handedness Inventory (Oldfield, 1971). Mental rotation
performance was measured using the Letter Rotation subtest LPS 7 from theLeistungs-Prüf-System(Horn, 1983), a well-established
paper–pencil test for mental plane rotation of single letters (test duration: 2 min). In addition, criteria of math talent, math and mean school grades (German grades: 1 = very good, 6 = not suffi-cient), the daily time spent on extracurricular math activities and other hobbies were documented.
2.4. Procedure
After the subjects were enrolled in the study, had their person-related data recorded and handedness surveyed, they practiced the
experimental mental rotation task outside of the scanner for 15 min (PC version; Borland Delphi 6.0). The program allowed the subjects to explore the task, the properties of the stimulus and the meaning of arrows (Fig. 2). For example, they could ac-tively rotate the object in the diverse directions by mouse-clicks to become familiar with the different positions and the effects of 90°rotations on the figure. The speed demands (i.e. the number of required mental rotations during the mental rotation phase) could be controlled to find out the maximum speed demand level which was subjectively experienced as still convenient. For further exploration, the stimulus was made available as a white cardboard object (12012060 mm3). During familiarization, subjects re-ceived feedback on the correctness of their responses and the rota-tion plane was changed three times during one trial, i.e. after each
Table 1
Subject characteristics and test scores: means (SD) and frequencies.
Math talent Gender
Talented subjectsn= 17 Control subjectsn= 17 p Male subjectsn= 17 Female subjectsn= 17 p
Gender m/f 8/9 8/9 1.000a
Math talent yes/no 8/8 9/9 1.000a
Handedness RH/LH/MH 14/1/2 15/0/2 0.596a 13/0/3 16/1/1 0.333a
Handedness Laterality Indexc 0.7 (0.6) 0.9 (0.4) 0.540b 0.8 (0.5) 0.9 (0.5) 0.528b
Age (years) 16.7 (1.1) 16.5 (0.9) 0.518b 16.5 (1.0) 16.7 (0.9) 0.463b
Extracurricular math activities (min/day) 64.1 (49.7) 14.3 (12.2) 0.001b 52.3 (56.6) 40.9 (34.1) 0.940b
Math graded 1.3 (0.6) 2.3 (1.1) 0.005b 1.6 (1.0) 1.9 (1.0) 0.347b
Total average graded 1.9 (0.5) 2.3 (0.5) 0.085b 2.1 (0.6) 2.1 (0.5) 0.772b
CFT-20R(IQ)e 118.2 (13.1) 110.1 (14.5) 0.106b 115.5 (15.7) 113.0 (13.1) 0.772b
LPS 7 (C score)f 8.5 (1.2) 7.9 (1.0) 0.160b 27.4 (4.3) 25.7 (5.2) 0.403b
Pre-scanning: mental rotation level 7.0 (2.4) 5.5 (1.0) 0.031b 7.2 (2.4) 5.4 (1.0) 0.017b
Mental rotation score (MRX) 0.88 (0.09) 0.72 (0.20) 0.018b 0.86 (0.14) 0.74 (0.18) 0.030b
Behavioral performance: means (SD).
aChi-square test. b Mann–Whitney test. c Oldfield (1971).
d German grades: 1 = very good (A grade), 6 = insufficient (F grade). eWeiß (2006).
f Horn (1983), C score: [mean = 5, SD = 2].
Fig. 2.Modified mental rotation paradigm. Shown are the control condition (no-rotation or pure maintenance condition) and the task condition ((no-rotation axis changing). The task condition was announced to the subject each time before the starting stimulus was presented.
Fig. 1.Object for mental rotation. The figure is constructed out of three small cubes in contrast to the original Shepard and Metzler (1971) objects which are constructed out of ten cubes.
single rotation. Subjects were informed about the control condition and the alternating sequence of true tasks and control tasks ap-plied during scanning. The total time in the scanner was about 35 min, including the preceding localizer and the subsequent T1-weighted structural brain scan. The assessment was completed by applying theCFT-20 Rpart 1 and theLPS 7after scanning. The
total duration of the examination was about 75 min.
2.5. Magnetic resonance image acquisition
Magnetic resonance image scanning was performed on a 1.5T MRI Scanner (Siemens Avanto, Erlangen, Germany) using a TIM 8-channel standard head coil. We acquired 500 T2-weighted, gra-dient echo planar imaging (EPI) scans including three initial dum-my scans that were discarded in order to achieve steady-state magnetization with the following parameters: slice-thick-ness = 3 mm; interslice gap = .3 mm; matrix size = 6464; field of view = 192192 mm2; echo time (TE) = 40 ms; repetition time (TR) = 2910 ms. Thirty-five transversal slices were acquired which covered the whole cerebral cortex but only the upper part of the cerebellum. In addition, we obtained a sagittal T1-weighted 3D-mprage sequence with 160 slices.
Scanning was comprised of 30 trials in the control condition and 30 trials in the task condition. The inter-block interval was jit-tered and varied randomly between 1.5 and 2.5 s. The scanning procedure always followed the sequence: localizer (1 min), mental rotation (6022 s = 22 min), MPRAGE (8 min). The task was pre-sented via video goggles (Nordic NeuroLab, Bergen, Norway) using PresentationÓ software (NeuroBehavioural Systems Inc., Albany/ California, USA; monitor resolution: 1024768 pixels). Subjects indicated their answers with the help of response grips (Nordic-NeuroLab, Bergen, Norway).
2.6. Behavioral data analysis
The effects of math talent and gender on MRX were tested using a bifactorial ANOVA andpost hoc T-tests for independent samples. Nonparametric tests (Mann–Whitney U-test,
v
2-test) were used to test group differences of non-normally distributed adjunctive mea-sures (e.g. school grades). Correlations ofMRX, other performance-related parameters and brain activation parameter estimates were analyzed by Pearson’s product-moment correlation. The signifi-cance level was set toa
= .05. Behavioral data were analyzed by SPSS (Version 17.0.1., German release).2.7. Neuroimaging data analysis
The preprocessing was performed by FSL software version 4.1.2 (FMRIB’s Software Library,www.fmrib.ox.ac.uk/fsl). Preprocessing included realignment with unwarping; slice timing correction using Fourier-space time-series phase-shifting; motion correction using MCFLIRT; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; registration to stan-dard space an EPI-template (resampled voxel size after registra-tion: 333 mm3); and smoothing with a 8-mm Gaussian kernel. The fMRI statistical analysis was done using Statistical Parametric Mapping 5 (SPM5, www.fil.ion.ucl.ac.uk/spm/). The hemodynamic response to each block was modeled by a canonical hemodynamic response function. The onset was defined by the occurrence of the starting stimulus and the modeled block com-prised the entire mental rotation phase, except for the final test (duration: 17 s). For each subject, parameter images for the con-trasts of each condition were generated and subjected to a sec-ond-level group effects analysis using a one-way ANOVA (within subject) with group membership as a between subject factor. In or-der to identify mental rotation related activation irrespective of the
group factors, we calculated the contrast of the task conditionvs.
the control condition (‘‘task effect’’). To identify effects of the group factors, the task effect was contrasted between the respective groups (i.e. grouptask interaction, ‘‘group effects’’).
To exclude potentially confounding effects of error-related pro-cesses, the correct trials were modeled separately from the error trials in the first-level analysis and the second-level analysis in-cluded correct trials only. Error trials were modeled as an addi-tional regressor of no interest. To further control for possible confounding effects of different amounts of error-related process-ing, the experimental task performance score was included as a covariate in all group effects analyses on thea priorigroup factors, i.e. math talent and gender. All analyses were conducted with a threshold of p<.001, uncorrected, and an extent threshold of
k= 10 adjacent voxels. Anatomical labeling of peak activation vox-els was done by the Masked Contrast Images (mascoi) tool for SPM (version 2.11; Reimold, Slifstein, Heinz, Mueller-Schauenburg, & Bares, 2006) with a secondaryp<.001. Specific brain regions were analyzed by the MARSeille Boîte À Région d’Intérêt (MarsBaR) extension of SPM (Brett, Anton, Valabregue, & Poline, 2002; www.nitrc.org/projects/marsbar/).
According to the findings of a meta-analysis of neuroimaging studies on mental rotation (Zacks, 2008), our main focus was on the bilateral PPC, i.e. inferior (IPL) and superior parietal lobule (SPL). With regard to the frontoparietal axis of general intellectual functioning (Jung & Haier, 2007), we also wanted to examine group effects on activations in the dorsolateral prefrontal cortices (DLPFC). Therefore, we defined bilateral prefrontal and parietal search volumes and extracted individual mean beta values from all voxels of each search volume as parameter estimates for the task-related activations in this area. The search volumes were masked by task effects from the total sample restricting this anal-ysis to task-related brain regions (Wake Forest University PickAtlas tool for SPM, release 2.4; Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Kraft, & Burdette, 2003). Multivariate analyses of covariance (MANCOVA) on the activation parameter estimates were performed with the experimental task performance score,
MRX, as a covariate and math talent, gender and experimental task performance as the group factors. In addition, correlation and regression analyses were performed on activation parameter esti-mates andMRX. To evaluate the relative impact of the three group factors on task-related brain activations in the selected brain areas, multiple linear regression analyses (method: stepwise) were per-formed on the activation parameters from the search volumes. Analyses including brain activation parameters were also per-formed by SPSS (Version 17.0.1., German release).
3. Results
3.1. Performance
As shown inTable 1, math talent and gender were stochastically independent. In addition, both group factors showed no effect on age, handedness distribution, nonverbal intelligence (CFT-20 R)
and two-dimensional mental letter rotation performance (LPS 7). MATH spent significantly more time per day on extracurricular mathematical activities (Mann–WhitneyU-test,p< .001), had
bet-ter math grades (p< .005), and a tendency towards better total average grades (non-significant trend,p< .085). No gender effects on adjunctive behavioral measures were obtained.
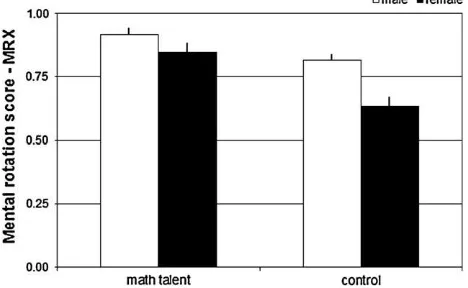
Fig. 3shows the group effects on the mental rotation perfor-mance score,MRX. Bifactorial univariate ANOVA confirmed main effects of the factors math talent [F(1,30) = 10.57, p<.003,
vs. CON and male vs. female subjects showed better mental rota-tion performance. Regarding the control task condirota-tion, the bifac-torial univariate ANOVA obtained a main effect for math talent [F(1,30) = 5.16,p<.030,
g
2= 0.15] indicating higher accuracy of MATH during the maintenance condition; no main effect of gender (p=.836) and no ‘‘talentgender’’ interaction effect (p=.653) were revealed.MRXwas correlated with the pre-scanningself-esti-mate of maximum mental rotation speed (r= 0.41,p<.017), men-tal letter rotation performance (r= 0.41, p<.016), math grades (r= 0.57, p<.001), and, in a non-significant trend, nonverbal
intelligence (r= 0.32,p=.062).
The stimulation task performance factor was retrospectively de-fined by a median split of the total sample based onMRX
(cut-off = 0.85, high/low performers: 17/17). The performance factor was significantly correlated with math talent [
v
2(1) = 5.765,p<.016] (5/17 MATH were low performers, 5/17 CON were high performers) but not with gender [
v
2(1) = 1.889, p=.169] (6/16 male subjects were low performers, 7/18 female subjects were high performers).For the total sample, error rates from the active control condi-tion were significantly lower than from the task condicondi-tion (Mean ± SD: baseline = 0.06 ± 0.07; task = 0.26 ± 0.19) [T(33) = 6.85, P< .001]. As a non-significant trend, mean error
rates from task trials with one change of the rotation axis were lower than for trials with three changes of the rotation axis [T(33) = 1.977,p=.056] thus indicating that the modified mental
rotation paradigm allows the manipulation of task difficulty in terms of cognitive load.
3.2. Task effects
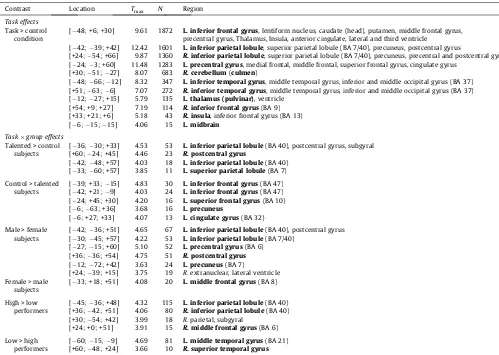
Mental rotation activated widespread parietal and frontal re-gions in the cerebral hemispheres, midbrain and also cerebellum (Table 2). The peak activation for the ‘‘taskvs.control condition’’ contrast was in the left IPL (Montreal Neurological Institute [MNI] template coordinates for the peak activation voxel:
X= 42;Y= +39;Z= +42). More specifically, the activated inferior parietal region comprised the supramarginal gyrus (SMG, Brod-mann area/BA 40/7) but not the angular gyrus (AG, BA 39).
3.3. Group effects
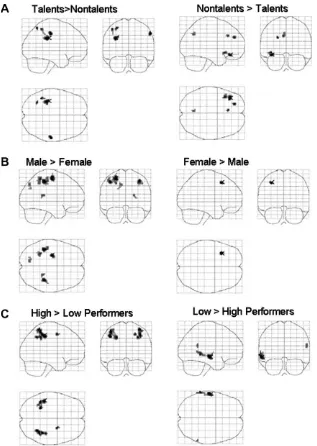
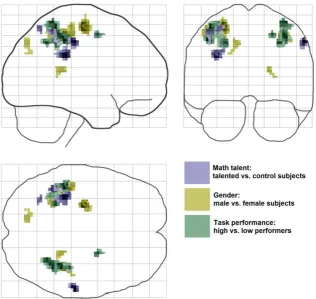
The effects of the two capability-related group factors, math tal-ent and gender, and the retrospectively defined task performance factor on task-related brain activations are shown inFig. 4;Fig. 5
shows a superposition of these effects (for anatomic labels of peak activation voxels seeTable 2). Behaviorally superior subjects (i.e. MATH, male subjects, and high performers) consistently showed higher activations in the left IPL (SMG, not AG) when compared to behaviorally inferior subjects. In addition, mathematically gifted subjects showed increased activation in the left SPL and the right postcentral gyrus. Additionally, male subjects showed activations in the left precuneus, left precentral and right postcentral gyrus. High performers also showed activations in the right IPL and the right middle frontal gyrus. In contrast, behaviorally inferior as compared to superior groups showed more diverse patterns of rel-atively increased activations either in left frontal (math talent, gen-der) or temporoparietal (experimental task performance) regions.
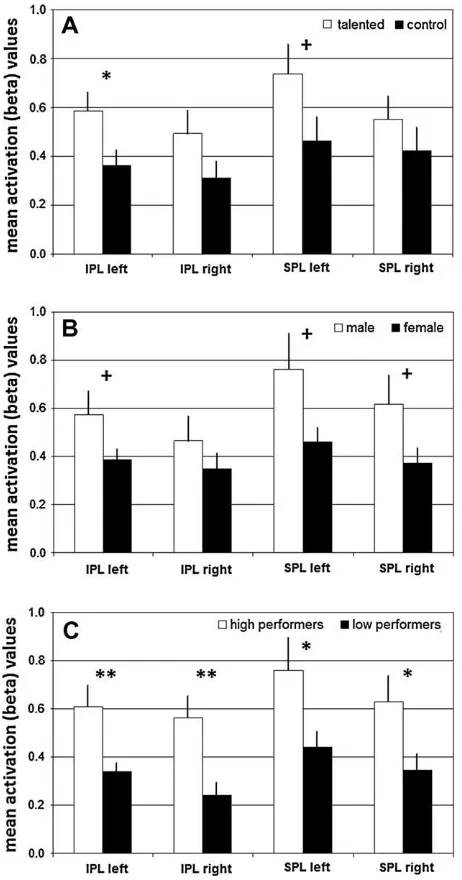
3.4. Activation parameters [Fig. 6 and 7]
Individual task-related activation parameter estimates (beta values) were extracted from the following frontal and parietal search volumes: left DLPFC corresponding to BA 9 (L.DLPFC, 60 voxels); right DLPFC (R.DLPFC, 29 voxels); left IPL (L.IPL, 383 vox-els); right IPL (R.IPL, 217 voxvox-els); left SPL (L.SPL, 199 voxvox-els); and right SPL (R.SPL, 171 voxels). The shown voxel numbers refer to the search volumes after masking by the task effect.
Activations in the left and right prefrontal search volumes showed no group effects [math talent: L.DLPFC: T(32) = 1.471,
p=.151; R.DLPFC: T(32) = 0.739; p=.465; gender: L.DLPFC: T(32) = 0.953, p=.348; R.DLPFC: T(32) = 0.640, p=.527; experi-mental task performance: L.DLPFC: T(32) = 0.736, p=.467; R.DLPFC:T(32) = 1.426,p=.163].
Fig. 6shows the group means of the parameter estimates ex-tracted from the parietal cortical search volumes for MATH and CON (panel A), male and female subjects (B), and high and low mental rotation performers (C). Repeated measures MANOVA including thea priorigroup factors, math talent and gender, and the four parietal activation parameter estimates (L.IPL, R.IPL, L.SPL, R.SPL) as dependent variables obtained no multivariate main or interaction effects (p> .196 for all effects).Post hoc univariate testing yielded a main effect of math talent on L.IPL activa-tion [F(1,30) = 5.518, p<.026,
g
2= 0.14] and near-significant trends towards main effects of math talent on L.SPL activa-tion [F(1,30) = 3.469,p=.072,g
2= 0.092] and of gender on L.IPL [F(1,30) = 3.999, p=.055,g
2= .101] and L.SPL activation [F(1,30) = 4.126, p=.051,g
2= 0.109]; no interaction effect was indicated. The univariate effects of talent and gender were omitted if MRX was included as a covariate into the model (p> .130 for alleffects). In contrast to thea priorigroup factors, the task perfor-mance group factor yielded a multivariate main effect on the pari-etal activation parameters [Wilks lambda= 0.702,F(4,29) = 3.079, p<.031]. In addition,post hocunivariate testing obtained effects of the performance factor on activation parameter estimates from each included parietal search volume [L.IPL: F(1,32) = 8.398,
p<.007,
g
2= .021; R.IPL:F(1,32) = 9.742,p<.004,g
2= 0.23; L.SPL:F(1,32) = 4.362,p<.045,
g
2= 0.12; R.SPL:F(1,32) = 5.084,p<.031,g
2= 0.14].MRX showed positive correlations with the activation parame-ter estimates from the parietal search volumes (L.IPL: r= 0.41,
p<.016; R.IPL: r= 0.40, p<.018; R.SPL: r= 0.39, p<.023) except for L.SPL the activation of which showed a near-significant trend (r= 0.34,p=.051). The scatter plots inFig. 7show the correlation ofMRXand task-related activations of the bilateral IPL; math talent and gender are also indicated. L.IPL activation was also positively correlated with the pre-scanning self-estimate of mental rotation speed (r= 0.363,p<.035).
To evaluate the relative contributions of the three performance factors to task-related brain activation, we performed stepwise multiple linear regression analyses on the activation parameters
Fig. 3.Experimental task performance score, MRX (group means, bars in graph are SEs of the mean). Bifactorial univariate ANOVA obtained main effects of math talent [F(1,30) = 10.57,p<.003,g2= 0.22] and gender [F(1, 30) = 6.7,p<.015,g2= 0.14]
indicating higher accuracy in the talented subjects and in the male subjects; no ‘math talentgender’ interaction effect was obtained,F(1, 30) = 1.22,p<.279.
from each of the four parietal search volumes. For all parietal activation parameters, the models only included the task performance factor and excluded the capability-related factors, math talent and gender (model parameters: L.IPL: b= 0.456,
corrected R2= 0.183,F(1,32) = 8.398,p<.007; R.IPL:b= 0.483,corrected
R2= 0.209, F(1,32) = 9.742, p<.004; L.SPL: b= 0.346, corrected R2= 0.092,F(1,32) = 4.362,p<.045; R.SPL:b= 0.370,corrected R2=
0.110, F(1,32) = 5.084, p<.031). The models explained only a small percentage of the variance of the task-related brain activations.
Multiple linear regression analyses for the prediction ofMRX in-cluded math talent (model parameter estimate: b= 0.475) and gender (b= 0.370) but none of the activation parameters as regres-sors [corrected R2= 0.321,F(2,31) = 8.813,p<.001]. A second
mod-el which exclusivmod-ely considered the four parietal activation parameters only included L.IPL activation as a regressor (b= 0.411,corrected R2= 0.143,F(1,32) = 6.487,p<.016).
4. Discussion
To examine the neural mechanisms underlying better cognitive performance, we combined the two established approaches to re-search on the brain–performance relationship by evaluating the ef-fects of math talent and gender as capability related group factors and experimental task performance on the brain activations during
a modified mental rotation task. As predicted, math talent and male gender were associated with higher mental rotation accuracy (hypothesis I). Mental rotation activated the bilateral IPL, among other parietal, frontal and temporal areas, and the left IPL was identified as a convergent zone of the effects of math talent, gender and task performance on task-related brain activations (hypothe-ses II–III). Activations in the left and right IPL and SPL were mostly explained by the effects of task performance, whereas math talent and gender were excluded from the multiple linear regression models (hypothesis IV). In the following discussion, we refer these findings to the neuroimaging evidence on mental rotation and to reported effects of math talent, gender and task performance. The discussion will be completed by relating NCP research to the research on neural efficiency.
4.1. Task effects
We proposed a novel mental rotation paradigm for NCP re-search. Mental rotation proper was separated from both the pre-ceding encoding of the initial position of the figure and from the final target-probe stimulus comparison test according to the prep-aration rotation paradigm (Bethell-Fox & Shepard, 1988; Jansen-Osmann & Heil, 2007; Lamm, Windischberger, Moser, & Bauer, 2007). In this paradigm response latency no longer represents a meaningful measure of mental rotation performance;
conse-Table 2
Total sample analyses: task effects and task by group effects. Contrast Location Tmax N Region Task effects
Task > control condition
[ 48; +6; +30] 9.61 1872 L. inferior frontal gyrus, lentiform nucleus, caudate (head), putamen, middle frontal gyrus, precentral gyrus, Thalamus, Insula, anterior cingulate, lateral and third ventricle
[ 42; 39; +42] 12.42 1601 L. inferior parietal lobule, superior parietal lobule (BA 7/40), precuneus, postcentral gyrus
[+24; 54; +66] 9.87 1360 R. inferior parietal lobule, superior parietal lobule (BA 7/40), precuneus, precentral and postcentral gyrus [ 24; 3; +60] 11.48 1283 L. precentral gyrus, medial frontal, middle frontal, superior frontal gyrus, cingulate gyrus
[+30; 51; 27] 8.07 683 R. cerebellum(culmen)
[ 48; 66; 12] 8.32 347 L. inferior temporal gyrus, middle temporal gyrus, inferior and middle occipital gyrus (BA 37) [+51; 63; 6] 7.07 272 R. inferior temporal gyrus, middle temporal gyrus, inferior and middle occipital gyrus (BA 37) [ 12; 27; +15] 5.79 135 L. thalamus (pulvinar), ventricle
[+54; +9; +27] 7.19 114 R. inferior frontal gyrus(BA 9) [+33; +21; +6] 5.18 43 R. insula, inferior frontal gyrus (BA 13) [ 6; 15; 15] 4.06 15 L. midbrain
Taskgroup effects
Talented > control subjects
[ 36; 30; +33] 4.53 53 L. inferior parietal lobule(BA 40), postcentral gyrus, subgyral [+60; 24; +45] 4.46 23 R. postcentral gyrus
[ 42; 48; +57] 4.03 18 L. inferior parietal lobule(BA 40) [ 33; 60; +57] 3.85 11 L. superior parietal lobule(BA 7) Control > talented
subjects
[ 39; +33; 15] 4.83 30 L. inferior frontal gyrus(BA 47) [ 42; +21; 9] 4.03 24 L. inferior frontal gyrus(BA 47) [ 24; +45; +30] 4.20 16 L. superior frontal gyrus(BA 10) [ 6; 63; +36] 3.68 16 L. precuneus
[ 6; +27; +33] 4.07 13 L. cingulate gyrus(BA 32) Male > female
subjects
[ 42; 36; +51] 4.65 67 L. inferior parietal lobule(BA 40), postcentral gyrus [ 30; 45; +57] 4.22 53 L. inferior parietal lobule(BA 7/40)
[ 27; 15; +60] 5.10 52 L. precentral gyrus(BA 6) [+36; 36; +54] 4.75 51 R. postcentral gyrus
[ 12; 72; +42] 3.63 24 L. precuneus(BA 7)
[+24; 39; +15] 3.75 19 R. extranuclear, lateral ventricle Female > male
subjects
[ 33; +18; +51] 4.08 20 L. middle frontal gyrus(BA 8)
High > low performers
[ 45; 36; +48] 4.32 115 L. inferior parietal lobule(BA 40) [+36; 42; +51] 4.06 80 R. inferior parietal lobule(BA 40) [+30; 54; +42] 3.99 18 R. parietal, subgyral
[+24; +0; +51] 3.91 15 R. middle frontal gyrus(BA 6) Low > high
performers
[ 60; 15; 9] 4.69 81 L. middle temporal gyrus(BA 21) [+60; 48; +24] 3.66 10 R. superior temporal gyrus
Peak activations.Location: Montreal Neurological Institute template coordinates;Tmax:T-value of peak activation voxel;N: number of clustering voxels that survived the
significance threshold ofp<.001, uncorrected (cluster size). The anatomic region of the peak activation voxel is written in bold (tentative Brodmann areas in parentheses). The experimental task performance score (MRX) was included as a covariate in the group effects analysis on math talent and gender. The T-maps are shown inFigs. 4 and 5.
quently, the classical mental rotation effect of a positive correla-tion between angular disparity of the stimulus to be rotated and the response latency can no longer be assessed (Shepard & Metzler, 1971). As the figure is physically absent during mental rotation, the modified paradigm addresses the maintenance (control task) and manipulation component (mental rotation proper) of visuospatial working memory without perceptual support. As intended, the ob-tained mean error rate during mental rotation proper in this study was moderate (26%) but still allowed superior subjects to excel (i.e. no floor/ceiling effect). Even in the subgroup with the worst perfor-mance (i.e. non-gifted female subjects), the mean error rate (36%) was below the mean error rates reported by others (>40%;O’Boyle et al., 2005; Weiss et al., 2003a,b).
Many fMRI studies demonstrated a specific role of the PPC for mental rotation (Alivisatos & Petrides, 1997; Booth et al., 2000; Co-hen et al., 1996; Gill, O’Boyle, & Hathaway, 1998; Halari et al., 2006, 2000; Harris & Miniussi, 2003; Hattemer et al., 2011;
Jaga-roo, 2004; Just, Carpenter, Maguire, Diwadkar, & McMains, 2001; Podzebenko, Egan, & Watson, 2002; Podzebenko, Egan, & Watson, 2005; Suchan, Botko, Gizewski, Forsting, & Daum, 2006; Weiss et al., 2003a,b; Zacks, 2008). These findings are in accordance with neuropsychological evidence (Farah, 1989). Current neurocognitive models for mental rotation consistently assign a key role to the PPC (Ecker, Brammer, & Williams, 2008; Jordan et al., 2002; Zacks, 2008). PPC is generally activated during the manipulation of infor-mation in working memory (Champod & Petrides, 2007, 2010). In addition, current models coherently include motor areas (precen-tral G., premotor area I, supplementary motor area/SMA) indicating either preparatory motor processes or a motor imagery component during mental rotation.
In keeping with these models, we obtained extended bilateral PPC activations during mental rotation. However, in our paradigm the peak activations were in the IPL instead of the SPL. SPL activa-tion was shown to be correlated with angular disparity in standard
Fig. 4.Group effects analyses on task-related brain activations. T-maps are shown as glass brains.Panel A: math talent;panel B: gender;panel C: experimental task performance factor (post hoc).Significance threshold:p<.001, uncorrected (height threshold:T(95) = 3.178248,N= 34); extent threshold:k= 10 clustering voxels. MNI template coordinates andTmax-values of the peak activation voxel, number of clustering voxels surviving the significance threshold, and anatomic regions are shown in Table 2. A superposition of these effects (left column) is shown inFig. 5.
mental rotation paradigms (Gogos et al., 2010) but we constantly used a rather small rotation angle of 90°. Several studies, including the first fMRI study on this paradigm (Cohen et al., 1996), dis-cussed the possibility that IPL activation during mental rotation in three-dimensional space reflects manual action imitation (mo-tor imagery) rather than visual imagery. Accordingly, a study employing repetitive transcranial magnetic stimulation provided evidence for a causal role of the IPL in motor imagery but not visual imagery (Pelgrims, Andres, & Olivier, 2009). Also, no IPL activation was obtained during a two-dimensional letter plane rotation which was presumed to comprise no motor imagery component (Podzebenko et al., 2002). In our own study, applying a mental ac-tion imitaac-tion strategy might have been suggested to the subjects by offering them the real cardboard object for evaluation during the pre-scan familiarization phase. In addition, our sample was comprised of non-standard subjects with a specific math talent and markedly higher mental rotation performance; both of these factors were related to increased activation of the IPL.
Consistent with one of the models (Ecker et al., 2008), we ob-served bilateral activations of the inferior temporal gyri indicating a role for higher-order visual object recognition. No SMA activation as a possible indicator of motor imagery was obtained, which is in line with two of the models (Ecker et al., 2008; Jordan et al., 2002). Contrasting with other recent studies, we did not find activations in hV5/MT+ or other occipital or temporooccipital regions ( Seur-inck, de Lange, Achten, & Vingerhoets, 2010). Notwithstanding the models, we obtained an extended activation of the left inferior frontal gyrus which might indicate a role for language-related pro-cessing (e.g. subvocal self-instruction).
4.2. Effects of math talent
Mental rotation performance was reported to be correlated with mathematical aptitude but other factors (e.g. gender) are
known to modulate this relation (Casey et al., 1995; Hyde, 2005; Nuttall, Casey, & Pezaris, 2004; Spelke, 2005). For example,Casey et al. (1995)reported that mental rotation performance was a bet-ter predictor of mathematical abilities (Scholastic Aptitude Test, SAT-M) than verbal performance (SAT-V) in all female and two of four male subsamples but not in talented males and male college students. However, one meta-analysis failed to confirm a relevant relation between visuospatial abilities and mathematical aptitude (Lubinski & Humphreys, 1990). Talent effects on mental rotation performance depend on the appropriate scaling of the task diffi-culty (no floor/ceiling effects) and might also be affected by the test modalities (e.g. paper–pencil vs. computerized test). For example, O’Boyle et al. (2005) reported an unexpected equally moderate mental rotation accuracy of mathematically gifted and control sub-jects during scanning (accuracy <60%, i.e. far off a floor/ceiling ef-fect). Thus, the complex relation between mental imagery capacity and mathematical precocity is still under debate.
Regarding the neural correlates of math talent,O’Boyle et al. (2005)reported increased activation of the right anterior cingulate and the left superior temporal gyrus during mental rotation as a correlate of mathematical giftedness. However, according to our definition, the obtained talent-specific pattern does not represent an NCP of mental rotation as it was not associated with better task performance. In contrast, the anterior cingulate is involved in er-ror-related processing and conflict monitoring (Baker & Holroyd, 2011; Cohen, Botvinick, & Carter, 2000), pain (Shackman et al., 2011), and depression (Bennett, 2011). Considering the high error rates during scanning, the obtained talent-specific brain response pattern might rather be related to motivational and emotional fac-tors with no or even a negative effect on task performance. Consis-tently, no mental rotation related activation was reported for the talent-specific activation areas in this study or in other studies (Ecker et al., 2008; Jordan et al., 2002; Zacks, 2008). A recent study of the same group (Prescott, Gavrilescu, Cunnington, O’Boyle, &
Fig. 5.Superposition of effects of math talent (blue), gender (yellow) and experimental task performance (green) on task-related brain activation (behaviorally superiorvs.
inferior subjects). For further details seeFig. 4. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Egan, 2010) showed enhanced frontoparietal brain connectivity during mental rotation (Shepard–Metzler paradigm) in mathemat-ically gifted vs. non-talented adolescents but again with equally low to moderate performance of both groups (accuracy: gifted 43%, controls 40%); thus, the same critical rational applies as to O’Boyle et al. (2005).
In the present study, mathematically gifted subjects showed superior performance during both the control condition and the mental rotation proper task indicating that math talent is related to both components of visuospatial working memory, maintenance and manipulation. To control for the potentially confounding ef-fects of different task performance, we excluded all error trials from second level analyses and included the accuracy score (MRX) as a covariate into the group effects analyses. Group effects analyses obtained increased activation in the left PPC (IPL, SPL) during mental rotation as a neural correlate of higher
mathemati-cal aptitude. This finding is consistent with a recent report of in-creased task-related (right) IPL activation during a reasoning task (Raven’s Advanced Progressive Matrices) in behaviorally superior mathematically gifted adolescents vs. controls (Desco et al., 2011). Of note, the mathematically gifted showed no better perfor-mance and also no IPL activation during a planning task (Tower of London). The functional data links to the evidence from morpho-metric studies on math aptitude: Professional mathematicians as compared to controls showed increased gray matter density in the bilateral IPL and gray matter density in the right IPL was pos-itively correlated with the time spent as an academician (Aydin et al., 2007). IPL activation was also reported for calculation and arithmetic approximation (Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999).
Fig. 6.Analysis of group effects on parameter estimates: Diagrams show the mean parameter estimates for task-related activations extracted from search volumes in the left and right inferior and superior parietal lobules (masked by task effects).
Panel A: math talent;Panel B: gender;Panel C: experimental task performance factor (post hoc). Results frompost hocunivariate analyses of the effects of math talent and
gender are indicated. For further details see Section3.p<.05,+p<.10. Fig. 7.
Scatter plots showing the correlation and linear regression of the experi-mental task performance score (MRX) as a regressor for the parameter estimates of task-related brain activation in the left (upper panel) and right (lower panel) inferior parietal lobule. The combined group memberships are indicated (male/ female: square/circle; control/talent: blank/filled).
4.3. Gender effects
To the best of our knowledge, this study is the first fMRI study to simultaneously compare the effects of two statistically indepen-dent capability-related group factors, talent and gender, on task-related brain activations. Mental rotation is supposed to show one of the strongest gender effects on cognitive performance ( Col-lins & Kimura, 1997; Kimura, 1992; Linn & Petersen, 1985; Masters & Sanders, 1993; Moore & Johnson, 2008; Quinn & Liben, 2008; Voyer & Hou, 2006; Voyer et al., 1995). However, in the majority of fMRI studies on mental rotation, no gender effect on task perfor-mance during scanning was obtained challenging the established use of this approach in order to examine the neural mechanisms behind gender-specific performance advantages (Butler et al., 2006; Christova, Lewis, Tagaris, Ugurbil, & Georgopoulos, 2008; Hugdahl, Thomsen, & Ersland, 2006; Jordan et al., 2002; Thomsen et al., 2000; Unterrainer et al., 2000; Weiss et al., 2003a,b). The equal behavioral outcomes probably did not result from the re-ported high error rates (mean response accuracies <60%), i.e. a floor/ceiling effect. For example,Butler et al. (2006)found no gen-der effect despite higher mean accuracy rates (>70%, pair-wise pre-sentation). Rather, the adaptation of the paradigm for fMRI use might have omitted features from the paper–pencil Vandenberg–
Kuse Mental Rotation Test (Peters & Battista, 2008; Peters et al.,
1995; Vandenberg & Kuse, 1978) which contribute to the gender effect. Actually, a series of behavioral studies indicated that stimu-lus characteristics (Jansen-Osmann & Heil, 2007; Neubauer, Berg-ner, & Schatz, 2010), time constraints (Goldstein, Haldane, & Mitchell, 1990; Peters, 2005/study I; but also seeMasters & Sand-ers, 1993), reduced number of items attempted (Voyer, 1997), and a reduced processing of mirrored as well as ‘occluded’ (i.e. partially concealed) distracter probes (Kerkman, Wise, & Harwood, 2000; Peters, 2005; Voyer & Hou, 2006) determine the effect size. In addi-tion, prior exposure to computer or video games, rather than task selection, was reported to predict the male advantage in mental rotation (Cherney, Jagarlamudi, Lawrence, & Shimabuku, 2003; Cherney & Neff, 2004) while training may diminish the effect ( Neu-bauer et al., 2010).
As a result of equal task performance, most of the reported gen-der-specific patterns of task-related brain activations do not repre-sent an NCP of mental rotation according to our definition (Butler et al., 2006; Christova et al., 2008; Jordan et al., 2002; Weiss et al., 2003a,b). Without supportive behavioral or self-report data, the possible role of gender-specific (but equally effective) cognitive strategies remains speculative (Butler et al., 2006). Similarly, corlations between sex steroid hormone levels and mental rotation re-lated brain activations remain unspecific unless they are rere-lated to task performance (Dietrich et al., 2001; Hugdahl et al., 2006; Schoning et al., 2007; Thomsen et al., 2000). Gender effects on the BOLD response might even artificially result from the available methods to detect activated voxels (Marcar, Loenneker, Straessle, Girard, & Martin, 2004). Reminding subjects of gender stereotypes before scanning has the potential to induce gender effects in terms of altered task-related brain activations in areas which are associ-ated with emotional and social stress processing (Krendl, Richeson, Kelley, & Heatherton, 2008). Conversely, one study found no gen-der-specific pattern of brain activation during mental rotation, although the expected effect on performance was obtained (Halari et al., 2006).
In the present study, gender affected mental rotation proper accuracy as expected. In contrast to math talent, gender did not yield an effect on stimulus maintenance (i.e. control condition) indicating that performance advantages in males exclusively relied on the manipulation component of this visuospatial working mem-ory task. This finding is in contrast with an EEG/ERP study on men-tal rotation (Yu et al., 2009) which reported a gender effect on the
early ERP of visual processing (400 ms after stimulus presentation, right frontal) but not at later stages during mental rotation proper (900–1000 ms, parietal). To control for the potentially confounding effect of task performance, we again excluded all error trials from the second level analyses and included the accuracy score (MRX) as a covariate in the group effects analyses. Group effects analyses re-vealed increased activation of the left IPL (and some other areas) as a correlate of male gender which is consistent with other func-tional neuroimaging (Weiss et al., 2003a,b) and volumetric studies (Frederikse, Lu, Aylward, Barta, & Pearlson, 1999). However, also a right hemisphere activation dominance of male subjects during mental rotation has been reported (Hattemer et al., 2011), and findings from brain volumetric gender studies on the IPL appear inconclusive (Chen, Sachdev, Wen, & Anstey, 2007; Takahashi, Ishii, Kakigi, & Yokoyama, 2010).
4.4. Effects of experimental task performance
In the vast majority of neurocognitive studies, the behavioral data (e.g. accuracy, response latencies) is largely ignored which raises the risk that important interindividual differences in the re-gional distribution of task-related brain activations (or functional connectivity) might be overlooked as a result of averaging ( Hug-dahl et al., 2006; Rypma et al., 2006; Thomsen et al., 2000). Con-versely, capability related brain activation patterns are interpreted as neural correlates of cognitive performance although no group effects on task performance existed and other unspecific group-related factors (e.g. stress) might have caused the effect.
We examined the impact of performance by analyzing the group effects of the experimental task performance factor, which was definedpost hocby a median-split based on the mental rota-tion proper accuracy score (i.e. corrected for maintenance perfor-mance). We obtained a pattern of increased bilateral IPL activation in high performers as the NCP of mental rotation proper. As previously mentioned, this region was strongly activated by the task. Using the standard paradigm, a bilateral activation of SPL (in-stead of IPL) was obtained as a positive neural correlate of mental rotation accuracy (Tagaris et al., 1996a, 1996b, 1997, 1998).
As predicted, the effect of the experimental task performance factor (bilateral IPL) showed a substantial overlap with the effects of math talent (left IPL/SPL) and gender (left IPL), so that the left IPL could be characterized as the convergent zone for capability and performance related effects on mental rotation related brain acti-vations. To evaluate the differential impact of these factors, we per-formed multiple linear regression analyses (method stepwise) on the activation parameters from four different posterior parietal re-gions. The models included task performance but excluded math talent and gender, thereby corroborating our hypothesis that the capability related effects can finally be explained by task perfor-mance effects.
This data strongly suggests that studies on capability related factors (e.g. gender, talent, diseases) should also address the possi-ble effects of task performance to avoid a misattribution of the ob-tained brain activation patterns to the group factor. Accordingly, one fMRI study reported the disappearance of a gender effect on brain activations during the planning phase of a complex problem solving task after carefully matching male and female subjects for their executive performance levels (Unterrainer et al., 2005). From an NCP perspective, the inclusion of both high-capability and aver-age subjects may facilitate the identification of NCPs by pushing upwards the average and the range of the individual levels of per-formance in untrained tasks.
with high cognitive load (Desco et al., 2011; Lee et al., 2006). Acti-vation of the bilateral IPL increased after practicing a working memory task (Kelly, Hester, Foxe, Shpaner, & Garavan, 2006) and repeating an arithmetic task (Krendl et al., 2008). PPC activations were shown to be related with manipulation processes in the working memory whereas middorsolateral frontal activations were correlated with the monitoring of the manipulated informa-tion (Champod & Petrides, 2007, 2010). A positive activation–per-formance relationship was obtained in the right IPL during the planning phase of a complex problem solving paradigm ( Unterra-iner et al., 2004). More generally, a role for the right IPL was ob-tained in decision making under uncertainty (Vickery & Jiang, 2009). Summarizing these findings, the parieto-frontal integration theory (P-FIT) of general intelligence (g-factor) assigns a key role to the IPL (Jung & Haier, 2007). However, regarding brain structure, the evidence for a relation of cortical thickness of IPL and intelli-gence seems inconsistent (Karama et al., 2011; Luders, Narr, Thompson, & Toga, 2009).
The IPL was also associated with higher-order visual action per-ception (e.g. action recognition, action intention, agency detection, self-other discrimination) and visuomotor action control (Bays, Singh-Curry, Gorgoraptis, Driver, & Husain, 2010; Chaminade & Decety, 2002; Claeys, Lindsey, De Schutter, & Orban, 2003; Clower et al., 1996; Clower, West, Lynch, & Strick, 2001; Fogassi et al., 2005; Fogassi & Luppino, 2005; Mattingley, Husain, Rorden, Ken-nard, & Driver, 1998; Preston & Newport, 2008; Rizzolatti, Ferrari, Rozzi, & Fogassi, 2006; Rizzolatti & Matelli, 2003; Singh-Curry & Husain, 2009; Uddin, Molnar-Szakacs, Zaidel, & Iacoboni, 2006). Le-sions of the left IPL may result in a pantomime recognition disorder and ideomotor limb apraxia (Joseph, 1990; Rizzolatti & Matelli, 2003).
4.5. NCP vs. neural efficiency
The Oxford Dictionary defines efficiency as ‘‘the ratio of the use-ful work performed by a machine or in a process to the total energy expended or heat taken in’’. Of note, in psychology better cognitive performance equals higher cognitive efficiency because subjects are generally tested under the condition of equal external re-sources (e.g. available time). Thus, searching for NCPs generally equals searching for neural correlates of cognitive efficiency. Intro-ducing effort or persistency as further internal resources (Larson et al., 1995; Neubauer & Fink, 2009) is acceptable only if indepen-dent data from the motivational dimension are provided.
In sharp contrast to NCPs, the term ‘‘neural efficiency’’ refers to the idea that more efficient brains need fewer neural resources (energy, volume, time) to achieve a defined cognitive aim ( Ander-son, 1995; Ertl & Schafer, 1969; Haier et al., 1988; Neubauer & Fink, 2009; Rypma et al., 2006). Strictly speaking, the neural efficiency approach does not assess the co-variance of brain-related mea-sures and cognitive performance, but rather the within-group var-iance of cognitive performance in subjects showing equal brain activations (same resources, different outcomes) or, conversely, the within-group variance of brain-related measures in equally performing subjects (same outcome, different resources). In the case of equal task performance (no floor/ceiling effect) increased task-related brain activations in highvs. low capability subjects
therefore has to be regarded as an indicator of lower (not higher) neural efficiency in the high capability subjects (Butler et al., 2006; Christova et al., 2008; Jordan et al., 2002; O’Boyle et al., 1995, 2005; Prescott, Gavrilescu, Cunnington, O’Boyle, & Egan, 2010; Weiss et al., 2003a,b). Evidently, in the reverse case of neg-ative activation–performance correlations the high-performing and low-activating subjects also show higher neural efficiency according to our definition.
Following the comprehensive review of Neubauer and Fink (2009), even the direction of the relationship between brain activa-tion and cognitive performance is far from being clear. The correla-tion seems to be strongly affected by task and sample characteristics and, furthermore, depends on the applied neuro-physiological measures (EEG, functional PET or fMRI) and the brain region under examination. The neural efficiency account initially relied on EEG data (Ertl & Schafer, 1969) but is now also supported by several neuroimaging studies (Neubauer & Fink, 2009). How-ever, many fMRI and functional PET studies reported positive brain–performance correlations, i.e. increased brain activations in the better performing subjects, which appears incompatible with the neural efficiency account (Boivin et al., 1992; Desco et al., 2011; Geake & Hansen, 2005; Gray, Chabris, & Braver, 2003; Haier & Benbow, 1995; Haier, White, & Alkire, 2003; Larson et al., 1995; Lee et al., 2006; Rypma et al., 2006; Rypma & Prabhakaran, 2009; Weiss et al., 2003a,b). Increasing the objective task demands also increases the task-related brain activations (Desco et al., 2011; Duncan et al., 2000; Gray et al., 2003; Jaeggi et al., 2003, 2007; Lee et al., 2006; Vannini et al., 2004).
Consistent with our own findings, negative brain–performance correlations were mostly reported for frontal brain areas (Jaeggi et al., 2003, 2007; Prat, Keller, & Just, 2007; Reichle, Carpenter, & Just, 2000; Ruff, Knauff, Fangmeier, & Spreer, 2003; Rypma, Berger, & D’Esposito, 2002; Rypma et al., 2006; Rypma & D’Esposito, 1999; Waiter et al., 2008). Some authors therefore suggested applying the neural efficiency account exclusively to the frontal lobes ( Neu-bauer & Fink, 2009; Toffanin, Johnson, de Jong, & Martens, 2007). However, considering the typical working memory and cognitive control functions of the frontal lobes, a negative brain–perfor-mance correlation in this defined brain area could alternatively be interpreted as a (positive) correlate of implicit executive func-tions which are increasingly activated with increasing task de-mands and with increasingly approaching the individual working memory capacity limits (Boivin et al., 1992; Gray et al., 2003; Jaeg-gi et al., 2007, 2003; Rypma et al., 2006; Rypma & Prabhakaran, 2009; Waiter et al., 2008; Weissman, Woldorff, Hazlett, & Mangun, 2002). For example, activation of the middorsolateral frontal lobes was shown to be correlated with the monitoring requirements and the memory load of a task (Champod & Petrides, 2007, 2010); acti-vation of the right ventral prefrontal cortex was shown to be cor-related with effort (Jansma, Ramsey, de Zwart, van Gelderen, & Duyn, 2007); and improving working memory performance re-sulted in reduced frontal lobe activation (Kelly et al., 2006; Ram-sey, Jansma, Jager, Van Raalten, & Kahn, 2004). Thus, frontal activation might be regarded as an individual neural indicator of a critically increasing task difficulty (or complexity) and, finally, decreasing task performance which results in a negative activa-tion–performance relationship in this brain region (Kelly & Garavan, 2005; Petersen, van Mier, Fiez, & Raichle, 1998).
4.6. Limitations
mance related neurocognitive processes in brain areas which are not primarily involved in mental rotation proper.
5. Conclusion
Three-dimensional mental rotation performance activated the bilateral IPL, and activation of this area was identified as a positive NCP of mental rotation. Mathematically gifted subjects and male subjects showed similar PPC activations and, consistently, better experimental task performance. The focus on NCP research should be set on data inherent to the experimental paradigm, i.e. perfor-mance and brain activations. However, the inclusion of subjects from special populations with presumably higher performance in the untrained experimental task may facilitate the search for its NCPs.
Acknowledgments
This study was granted by the Karg-Foundation for the Advancement of Gifted Children, Frankfurt/Main (http:// www.karg-stiftung.de). S.S. received a grant from the Deutsche
Forschungsgemeinschaft(SFB-TR3). We wish to thank all our
partic-ipants who volunteered for this study. Further thanks to Mrs. Beate Newport for providing important technical support.
References
Alivisatos, B., & Petrides, M. (1997). Functional activation of the human brain during mental rotation.Neuropsychologia, 35(2), 111–118.
Anderson, B. (1995). G explained.Medical Hypotheses, 45(6), 602–604.
Aydin, K., Ucar, A., Oguz, K. K., Okur, O. O., Agayev, A., Unal, Z., et al. (2007). Increased gray matter density in the parietal cortex of mathematicians: A voxel-based morphometry study.AJNR American Journal of Neuroradiology, 28(10), 1859–1864.
Baker, T. E., & Holroyd, C. B. (2011). Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200.Biological Psychology, 93(4), 468–487.
Bays, P. M., Singh-Curry, V., Gorgoraptis, N., Driver, J., & Husain, M. (2010). Integration of goal- and stimulus-related visual signals revealed by damage to human parietal cortex.Journal of Neuroscience, 30(17), 5968–5978.
Bell, E. C., Willson, M. C., Wilman, A. H., Dave, S., & Silverstone, P. H. (2006). Males and females differ in brain activation during cognitive tasks.Neuroimage, 30(2), 529–538.
Bennett, M. R. (2011). The prefrontal-limbic network in depression: Modulation by hypothalamus, basal ganglia and midbrain.Progress in Neurobiology, 87(1), 25–34.
Bethell-Fox, C. E., & Shepard, R. N. (1988). Mental rotation – Effects of stimulus complexity and familiarity.Journal of Experimental Psychology-Human Perception and Performance, 14(1), 12–23.
Boivin, M. J., Giordani, B., Berent, S., Amato, D. A., Lehtinen, S., Koeppe, R. A., et al. (1992). Verbal fluency and positron emission tomographic mapping of regional cerebral glucose metabolism.Cortex, 28(2), 231–239.
Booth, J. R., MacWhinney, B., Thulborn, K. R., Sacco, K., Voyvodic, J. T., & Feldman, H. M. (2000). Developmental and lesion effects in brain activation during sentence comprehension and mental rotation.Developmental Neuropsychology, 18(2), 139–169.
Brett, M., Anton, J. -L., Valabregue, R., & Poline, J. -B. (2002). Region of interest analysis using an SPM toolbox (Abstract 10511. In Presented at the 8th international conference on functional mapping of the human brain, June 2–6, 2002, Sendai/Japan) [available on CD-ROM].NeuroImage,16(2Suppl. 1). Butler, T., Imperato-McGinley, J., Pan, H., Voyer, D., Cordero, J., Zhu, Y. S., et al.
(2006). Sex differences in mental rotation: Top-down versus bottom-up processing.Neuroimage, 32(1), 445–456.
Casey, M. B., Nuttall, R., & Benbow, C. P. (1995). The influence of spatial ability on gender differences in mathematics college entrance test-scores across diverse samples.Developmental Psychology, 31(4), 697–705.
Chaminade, T., & Decety, J. (2002). Leader or follower? Involvement of the inferior parietal lobule in agency.NeuroReport, 13(15), 1975–1978.
Champod, A. S., & Petrides, M. (2007). Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes.Proceedings of the National Academy of Sciences of the United States of America, 104(37), 14837–14842.
Champod, A. S., & Petrides, M. (2010). Dissociation within the frontoparietal network in verbal working memory: A parametric functional magnetic resonance imaging study.Journal of Neuroscience, 30(10), 3849–3856. Charlot, V., Tzourio, N., Zilbovicius, M., Mazoyer, B., & Denis, M. (1992). Different
mental imagery abilities result in different regional cerebral blood flow activation patterns during cognitive tasks.Neuropsychologia, 30(6), 565–580.
Chen, X., Sachdev, P. S., Wen, W., & Anstey, K. J. (2007). Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel-based morphometric study.Neuroimage, 36(3), 691–699.
Cherney, I. D., Jagarlamudi, K., Lawrence, E., & Shimabuku, N. (2003). Experiential factors in sex differences on mental rotation.Perceptual and Motor Skills, 96(3 Pt 2), 1062–1070.
Cherney, I. D., & Neff, N. L. (2004). Role of strategies and prior exposure in mental rotation.Perceptual and Motor Skills, 98(3 Pt 2), 1269–1282.
Christova, P. S., Lewis, S. M., Tagaris, G. A., Ugurbil, K., & Georgopoulos, A. P. (2008). A voxel-by-voxel parametric fMRI study of motor mental rotation: Hemispheric specialization and gender differences in neural processing efficiency.
Experimental Brain Research, 189(1), 79–90.
Claeys, K. G., Lindsey, D. T., De Schutter, E., & Orban, G. A. (2003). A higher order motion region in human inferior parietal lobule: Evidence from fMRI.Neuron, 40(3), 631–642.
Clower, D. M., Hoffman, J. M., Votaw, J. R., Faber, T. L., Woods, R. P., & Alexander, G. E. (1996). Role of posterior parietal cortex in the recalibration of visually guided reaching.Nature, 383(6601), 618–621.
Clower, D. M., West, R. A., Lynch, J. C., & Strick, P. L. (2001). The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum.Journal of Neuroscience, 21(16), 6283–6291.
Cohen, J. D., Botvinick, M., & Carter, C. S. (2000). Anterior cingulate and prefrontal cortex: Who’s in control?Nature Neuroscience, 3(5), 421–423.
Cohen, M. S., Kosslyn, S. M., Breiter, H. C., DiGirolamo, G. J., Thompson, W. L., Anderson, A. K., et al. (1996). Changes in cortical activity during mental rotation. A mapping study using functional MRI.Brain, 119(Pt 1), 89–100.
Collins, D. W., & Kimura, D. (1997). A large sex difference on a two-dimensional mental rotation task.Behavioral Neuroscience, 111(4), 845–849.
Deary, I. J., Penke, L., & Johnson, W. (2010). The neuroscience of human intelligence differences.Nature Reviews Neuroscience, 11(3), 201–211.
Dehaene, S., Spelke, E., Pinel, P., Stanescu, R., & Tsivkin, S. (1999). Sources of mathematical thinking: Behavioral and brain-imaging evidence. Science, 284(5416), 970–974.
Desco, M., Navas-Sanchez, F. J., Sanchez-Gonzalez, J., Reig, S., Robles, O., Franco, C., et al. (2011). Mathematically gifted adolescents use more extensive and more bilateral areas of the fronto-parietal network than controls during executive functioning and fluid reasoning tasks.Neuroimage, 57(1), 281–292.
Dietrich, T., Krings, T., Neulen, J., Willmes, K., Erberich, S., Thron, A., et al. (2001). Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI.Neuroimage, 13(3), 425–432. Duncan, J., Seitz, R. J., Kolodny, J., Bor, D., Herzog, H., Ahmed, A., et al. (2000). A
neural basis for general intelligence.Science, 289(5478), 457–460.
Ecker, C., Brammer, M. J., & Williams, S. C. (2008). Combining path analysis with time-resolved functional magnetic resonance imaging: The neurocognitive network underlying mental rotation.Journal of Cognitive Neuroscience, 20(6), 1003–1020.
Ertl, J. P., & Schafer, E. W. (1969). Brain response correlates of psychometric intelligence.Nature, 223(5204), 421–422.
Farah, M. J. (1989). The neural basis of mental imagery.Trends in Neurosciences, 12(10), 395–399.
Fogassi, L., Ferrari, P. F., Gesierich, B., Rozzi, S., Chersi, F., & Rizzolatti, G. (2005). Parietal lobe: From action organization to intention understanding.Science, 308(5722), 662–667.
Fogassi, L., & Luppino, G. (2005). Motor functions of the parietal lobe.Current Opinion in Neurobiology, 15(6), 626–631.
Frederikse, M. E., Lu, A., Aylward, E., Barta, P., & Pearlson, G. (1999). Sex differences in the inferior parietal lobule.Cerebral Cortex, 9(8), 896–901.
Geake, J. G., & Hansen, P. C. (2005). Neural correlates of intelligence as revealed by fMRI of fluid analogies.Neuroimage, 26(2), 555–564.
Gill, H. S., O’Boyle, M. W., & Hathaway, J. (1998). Cortical distribution of EEG activity for component processes during mental rotation.Cortex, 34(5), 707–718. Gogos, A., Gavrilescu, M., Davison, S., Searle, K., Adams, J., Rossell, S. L., et al. (2010).
Greater superior than inferior parietal lobule activation with increasing rotation angle during mental rotation: An fMRI study.Neuropsychologia, 48(2), 529–535. Goldstein, D., Haldane, D., & Mitchell, C. (1990). Sex differences in visual-spatial ability: The role of performance factors.Memory & Cognition, 18(5), 546–550. Grabner, R. H., Neubauer, A. C., & Stern, E. (2006). Superior performance and neural
efficiency: The impact of intelligence and expertise.Brain Research Bulletin, 69(4), 422–439.
Gray, J. R., Chabris, C. F., & Braver, T. S. (2003). Neural mechanisms of general fluid intelligence.Nature Neuroscience, 6(3), 316–322.
Haier, R. J. (2009). Neuro-intelligence, neuro-metrics and the next phase of brain imaging studies.Intelligence, 37(2), 121–123.
Haier, R. J., & Benbow, C. P. (1995). Sex differences and lateralization in temporal lobe glucose metabolism during mathematical reasoning. Developmental Neuropsychology, 11(4), 405–414.
Haier, R. J., Siegel, B. V., Nuechterlein, K. H., Hazlett, E., Wu, J. C., Paek, J., et al. (1988). Cortical glucose metabolic-rate correlates of abstract reasoning and attention studied with positron emission tomography.Intelligence, 12(2), 199–217. Haier, R. J., White, N. S., & Alkire, M. T. (2003). Individual differences in general
intelligence correlate with brain function during nonreasoning tasks.
Intelligence, 31(5), 429–441.
Halari, R., Sharma, T., Hines, M., Andrew, C., Simmons, A., & Kumari, V. (2006). Comparable fMRI activity with differential behavioural performance on mental rotation and overt verbal fluency tasks in healthy men and women.
Experimental Brain Research, 169(1), 1–14.